Inspiration
Tuberculosis (TB) is the leading killer of people in the world and a major cause of deaths related to antimicrobial resistance. According to the WHO (World Health Organization), more than 10.4 million TB cases were reported in 2017. Among them, 1.7 million deaths were reported. Epidemiological studies show that 95% of the cases are reported in countries with a low GDP (Gross Domestic Product). Even if TB is almost eradicated in Europe, it remains a major public health issue in most parts of the world.
Tuberculosis: cause, evolution and treatment
The disease's agent is Mycobacterium tuberculosis. It's easily transmitted by air through droplets from an infected person to a healthy one. An untreated patient can transmit the disease to up to 10-15 individuals per year. Most of the time, after infection, the bacteria travel through the respiratory system. Three forms of TB are currently reported:
- The most common one is pulmonary TB. M. tuberculosis homes in the lungs, destroys the tissue and creates a cavity.
- The second one is an extra-pulmonary form. M. tuberculosis spreads to other tissues of the human body such as kidneys, lymph nodes, nervous system etc.
- The last form is a combination of the two previously mentioned ones.
 Courtesy of Stewart Cole/EPFL
Courtesy of Stewart Cole/EPFL
Currently, an antibiotics cocktail composed of Rifampicin, Ethambutol, Pyrazinamide and Isoniazide is the first-class treatment for TB. People at risk are the elders, the malnourished, the ones who suffer from immunodepression and people that live in disadvantaged socio-economic areas. The BCG (Bacillus Calmette–Guérin) vaccine is primarily used against TB. The vaccine is made from an attenuated strain of Mycobacterium bovis. However, over the last two decades, the vaccine's efficiency got controversial because of its unstable rate of protection. Nowadays, the BCG vaccine is no longer mandatory in most parts of the world.
Tuberculosis and multi-drug resistance
Some TB patients can develop drug resistance. Thus, they can’t be properly treated with current methods. Nowadays, two main drug resistant strains are reported:
- MDR-TB for multidrug resistant-TB. That strain is resistant to the two most powerful antibiotics: Isoniazid and Rifampicin.
- XDR-TB for extensively drug resistant-TB. That strain is resistant to the previously mentionned antibiotics, Fluoroquinolone and second-line antibiotics such as Kanamycin and Amikacin.
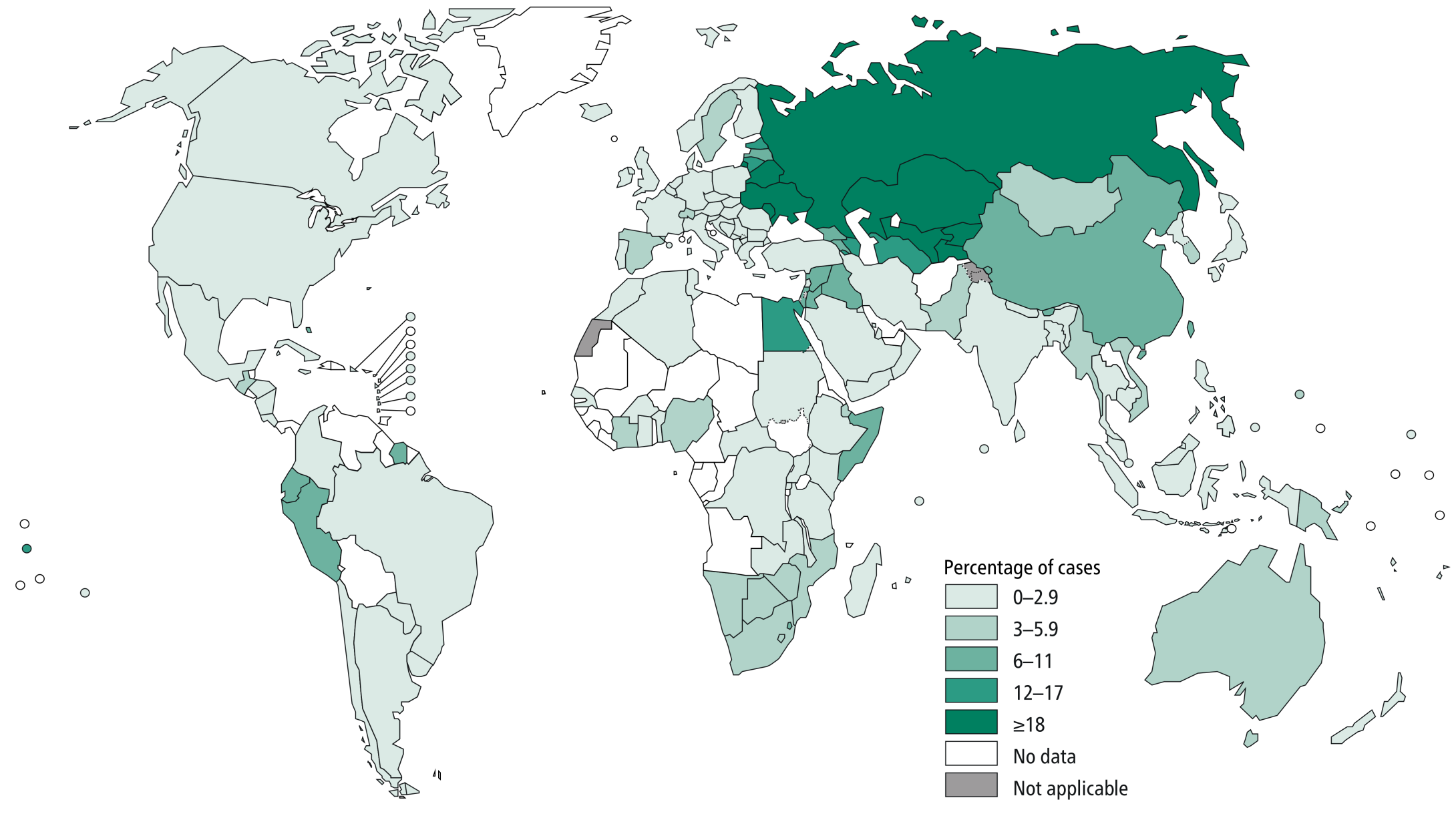
According to the WHO, 600 000 MDR-RR (Rifampicin Resistant)-TB and 8000 XDR-TB cases were reported worldwide in 2016. Currently, only 30% of XDR-TB patients recovered after a successful treatment. Thus, developing new diagnostics and treatment procedures for these new forms is essential to avoid TB resurgence. The world map below (courtesy of the WHO) shows the distribution of MDR-RR reported cases around the world in 2017.
Tuberculosis and diagnostics
Nowadays, TB diagnostic is mainly based on X-RAY to detect tuberculous lesions. The X-RAY is then followed by sample collection such as sputum or tissue biopsy. The sample is analyzed by microscopy to identify Acido/Alcalo resistant Bacillus. Then, it's cultivated using solid (Löwenstein–Jensen) or liquid (Middlebrook broth) mediums to detect M. tuberculosis. However, considering that the given bacterium has a slow growth rate, it may take up to 1 week to identify it. Once confirmed, molecular analysis (polymerase chain reaction etc.) and antibiograms are carried out to identify the strain's sensitivity and eventual drug-resistance form. Tests can also be done using blood samples to detect antibodies specific to the Mycobacteria phylum. However, the procedure is inaccurate and can lead to false negative results.
It doesn’t look like a big deal, but all of these procedures require expensive equipment, qualified personnel for data analysis and can take quite a lot of time. Indeed, in emerging countries, patients that come to the hospital to be tested, don’t always wait for the result because of the long delay and the geographic position of the hospital from their home. Therefore, patients that are infected return to their normal routine and take the risk of infecting other people and thus, disseminating the disease.
Tuberculosis and culture
TB had and still has a real impact in our everyday culture. As a worldwide disease, TB is present in every culture and has different meanings and interpretations throughout the generations. For a long time, TB was also known as the romantic disease. Many artistic figures including John KEATS (poet), Frederic CHOPIN (composer) and Edvard MUNCH (artist) either had the disease or were close to others who did. At the time, a belief was worn and said that TB assisted artistic talent. It enriched one's creativity.
In 1886, Edvard MUNCH painted the The sick child (picture on the right). It represented his sister as she was dying of TB. The disease is not only present in the field of art but also in Science, Politics etc. Great figures in history - such as Voltaire (writer), Nelson MANDELA (former president of South-Africa) and Erwin SCHRÖDINGER (physicist) - had TB.
 Courtesy of EdvardMunch.org
Courtesy of EdvardMunch.org
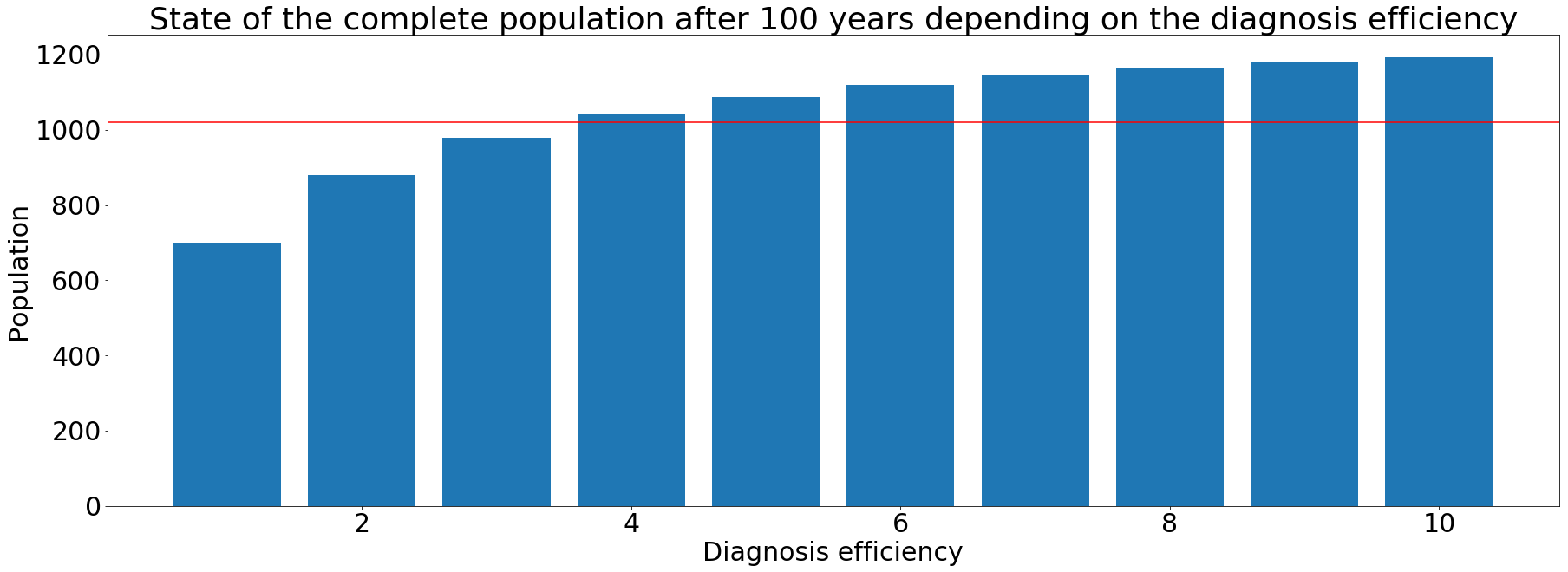
Epidemiological model
Our model aims to show the impact of an effective TB diagnostics test and some other variables on a given population. Our main inspiration was the model created by Abu-Raddad et al. The World Health Organization (WHO) participated in the elaboration of this model using known data (Find out more)
Description
I WANT TB FREE aims to improve existing diagnostics procedures that are relatively expensive and time consuming. After extensive research and interviews with TB specialists, we became aware of an additional issue of the current diagnostics procedures: limited accessibility in most parts of the world. Current methods rely mainly on blood samples and require expensive equipment (maintenance, infrastructure etc.). The limited access leads to a lack of treatment. These shortcomings lead to an increase in the proliferation of this disease worldwide. The development of a rapid and accessible diagnosis would greatly decrease TB cases.
The procedure took shape with the help of doctors, TB specialists and biologists.
Our diagnostics procedure (picture below) revolves around three main aspects:
- Cell lysis and total genomic DNA extraction from sputum samples.
- DNA specific amplification by PSR (Polymerase spiral reaction).
- Mycobacterium tuberculosis DNA detection using cellulose membranes and colorimetric protein recognition.
The procedure does not require any specific/expensive equipment. Our main goal is to make it fast, reliable and accessible to everyone. The PSR system is the main core of the procedure. Its main virtue is that it allows an isothermal DNA amplification. Unlike the PCR, it does not require a thermal cycler, but only a heater or a water bath. Another feature is that the PSR is relatively timesaving. Upon sample collection, DNA amplification takes about an hour and a half on average. This prevents the potential patient from going back home and putting his/her surroundings in danger.
Diagnostics procedure
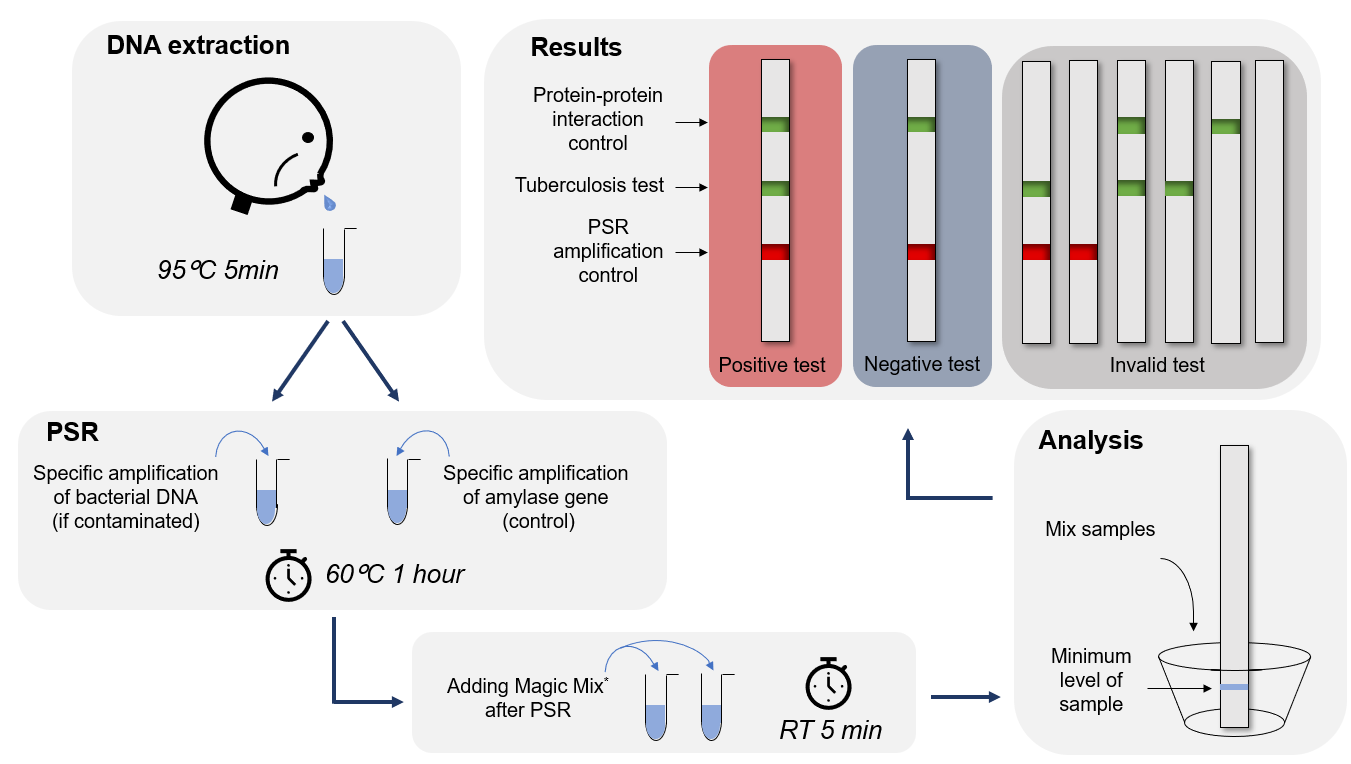 * The magic mix is composed of the fusions proteins we designed. These include TAL1, TAL2, TAL3 and P3-N1-Green chromoproteins.
* The magic mix is composed of the fusions proteins we designed. These include TAL1, TAL2, TAL3 and P3-N1-Green chromoproteins.
We translated our kit manual into several languages (Français, English, Español, العربيّة, Românesc, Italiano, русский, ǑहÛदȣ). Check it out
Kit components
The I WANT TB FREE procedure revolves around four major steps.
Bacterial lysis and DNA extraction
The bacterial lysis is made with a buffer and a variation of temperatures that both allow the extraction of the DNA by destabilizing the cell membrane.
PSR (Polymerase spiral reaction)
The DNA polymerase must be capable of polymerizing DNA strands at a relatively low temperature (or an ideal temperature of 60°C), so we can provide a procedure that does not require relatively expensive heating equipment.
The DNA polymerase is optimized, stabilized and designed to amplify the DNA fragment that will be then detected on cellulose membranes.
Following the PSR, TALENs will be added into the sputum sample. Two TALs were designed and coupled with two different chromoproteins. The chromoproteins help differentiate the two amplified genes, visible by the appearance of bands of different colors. The first TAL recognizes the human amylase amplified DNA. The second TAL recognizes the M. tuberculosis amplified DNA.
Vitrification
Vitrification stabilizes all the produced enzymes. It uses antigens that create a protective layer around each enzyme.
Cellulose membrane and strips
The cellulose strips are the detection component. We implemented three types of strips. Each one of them detects different molecules in the sputum sample.
Strip 1: Contains a methylase protein coupled to a cellulose binding domain (CBD). The complex could recognize the amplified DNA fragment of the human amylase gene. Strip 1 serves as the procedure's positive control. It ensures correct sampling and loading.
Strip 2: Contains a methylase protein coupled to a cellulose binding domain (CBD). The complex could recognize the amplified DNA fragment of M. tuberculosis. Strip 2 serves is the main detection component.
Strip 3: Contains a protein/protein interaction complex: TolA3/PIII-N1. The TolA3 protein is coupled to a CBD. The PIII-N1 protein is coupled to a chromoprotein. The complex serves as an interaction control. It ensures that the chromoprotein functions only when the interaction of the protein with which it is coupled and its partner.
From the very beginning of our project, we have thought a great deal about the human practices. In order to make our project as useful as possible we tried to shape it and develop our scientific approach according to new insights that we gained from specialists, medical professionals and the general public. How did we integrate human practices and how did it shape our project?


