Sensitive, specific and early diagnosis is a crucial step to study and cure a pathology with the right tools and timing. In addition to increasing the percentage of treatment success - the earlier a disease is detected, the more efficient the treatment will be. Early diagnosis is very cost effective as it can prevent misdiagnosis of some diseases, and unnecessary costly, invasive, or painful procedures or medications - in the case of neurodegenerative diseases, patients often start antidepressant medications before a correct diagnosis.[2] Obviously, in some cases, an early diagnosis reduces the potential diseases complications.
Moreover, the possibility to face the patients with this difficult statement in the earliest stage of the disease course can offer timely practical informations, advices and support for them and their family. Some drugs or non-drugs medications may be proposed to the patients in order to improve their quality of life. A specific and sensitive early diagnosis can open the doors to better patient management and patient care, because the treatment would be adjusted to the diagnosis instead of trying a treatment and waiting for the effects, without knowing if it is going to fit the patient well. That way, more informations about the disease evolution and about the efficiency of the given treatments can be gathered as soon as possible.
Today, we are able to detect a pregnancy in the first month, a high cholesterol level before it becomes dangerous, a deficiency, an inflammatory response or even diabetes with a few blood drops. However, some pathologies diagnosis can remain tricky, especially at an early stage in the disease course. This can be due to low awareness of disease signs and symptoms, thus people don’t think it is necessary to visit a doctor, while the disease is secretly proliferating. Some people put off doctor’s visit because they are afraid of what they may find [3]. Sometimes, there is also a delay that can occur in getting appointments with professionals.
If the diagnosis is made at an early stage, some problems might remain unsolved. Some diseases are difficult to observe because of their complex and hidden biomarkers. Some have a too low quantity of biomarkers, so that they can’t be detected. Moreover, an insufficient amount of the target biomarker can lead to the necessity of harvesting too large sample volume, involving invasive techniques, undermining patient’s well-being. Hence new diagnosis methods have to be investigated.
For some diseases, a too late detection of the disease results in late patient care, thus in lower likelihood of survival or recover, greater morbidity and higher costs of care, leading to avoidable deaths and disability from the disease.[1]
Different body fluids are at our disposal when it comes to biomarkers search or use in diagnosis. However, blood remains in the vast majority of cases the gold standard for human body sampling. That “choice” is justified as biomarkers blood concentration is statistically associated with most of the diseases, however blood sampling and even more for CSF present disadvantages from a practical standpoint. Recent investigations are shining a new light on the interest of other body fluids such as saliva and tears [12].
Tears allow humans to share their emotions, but their composition is also really interesting! A teardrop is composed of three layers [13]:
Figure 1: Animation of the attractive general composition of a teardrop, highlighting the medical potential use of them for diagnosis.
The use of saliva and tear fluid could allow us to avert invasive procedures, decrease the risk, pain and cost thus increasing the acceptance by patients. Even if tear fluid has no direct connection to the brain or other organs, the presence of both ocular (dry eye syndrome, diabetic retinopathy) and systemic diseases (cancer, multiple sclerosis, cystic fibrosis, Parkinson’s disease) biomarkers has been assessed in tear fluid [10]. Tears are a way less complex fluid than blood, as there are almost no cells, and way less different biomarkers, allowing us to get our way around a lot of issues.
As for neurodegenerative diseases and especially Parkinson’s disease, alpha-synuclein is present in tears, both in its physiological and oligomeric forms. With its prominent role in PD’s pathogenesis and its ability to be excreted by neurons, its presence is most likely due to nerve terminals of neurons innervating lacrimal glands that are located in the brainstem [13]. It has been shown that total alpha-synuclein levels in basal tears were decreased in PD patients, whereas oligomeric alpha-synuclein and the oligomeric/total alpha-synuclein ratio were both increased [13].
Figure 2: Advantages and disadvantages of different body fluids for biomarker research in PD. [13]
The proposition of the iGEM Grenoble team is to develop a device able to detect small amounts of biomarkers in small volumes such as tears. By doing so, their exploitation as biological sample for diagnosis would be allowed. This sensitive and specific detection method will be combined with a user-friendly device, operable by clinicians, medical doctors, and maybe by patients or the wider public on the long term. The device will also be cost-effective and therefore attractive for industries as well as research laboratories.
Moreover, due to the utilization of aptamers as system receptors, it is really flexible, because the process could be easily adapted to the detection of other pathologies in tears: allergies, vitamin deficiencies… Aptamers are short sequences of nucleic acids, highly specific and cost effective, they act like antibodies and begin to be widely used in the diagnosis world.
The team think that the accomplishment of all those goals would likely lead to a major breakthrough in the field of diseases diagnosis and diagnosis automating. In the near and the distant future, we can imagine that future design and development of new, more selective and effective therapies for individual patient can be helped with this change in diagnosis, giving rise to the personalized medicine.
NeuroDrop is a new synthetic biology detection device that includes several biological and engineered techniques in order to have a precise and time-saving tool.
Figure 3 Overview of the main engineered and biological parts of the system. Aptamers are single-stranded nucleic acid oligonucleotides. They have a strong affinity and specificity for several targets: cells, proteins, small organic molecules, ions. On the extracellular surface of our COMP protein is located a non-natural amino-acid: the azido phenylalanin. COMP proteins are fused at their C-terminal ends to a subunit of the adenylate cyclase of Bordetella pertussis: either T18 or T25. Then the cAMP molecules bind to the catabolite activator protein (CAP) and form a complex able to induce gene expression. In our system, we used this phenomenon to create our reporter plasmid, consisting of a CAP-responsive promoter driving expression of the NanoLuciferase.
The biological aspect is focused on the creation of a bacterial biosensor that detect small amounts of biomarkers present in small volumes. In order to achieve this goal, we used aptamers as specific and sensitive receptors. They are short nucleic acid sequences that catch specific molecules like antibodies do.
Our bacterial biosensor detects extracellular biomarkers, therefore the receptors have to be expressed at the external membrane of the bacterium.
To ensure that the biomarker recognition by the specific aptamer results in a fast and strong signal in the bacteria, we took advantage of the well-known Bacterial Adenylate Cyclase Two Hybrid (BACTH) method and improved it to fit with our specific system. Our biosensor COMBs are fused at their C-terminal domains to one of the two sub-part of the adenylate cyclase (AC) of Bordetella pertussis, either T25 or T18.
To detect the AC enzymatic activity, a Nanoluciferase reporter gene is located downstream a cAMP-CAP dependant lactose promoter. When the activity of adenylate cyclase enzyme is recovered, it produce high amounts of cAMP molecule. This molecule diffuses from the periplasm to the cytoplasm of the bacteria and interact with a Catabolite Activator Protein (CAP). The resulting complexes will then interact with the lactose promoter and induce the expression of the reporter gene, hence the Nanoluciferase enzyme is produced and the bacteria can now start to emit light in presence of the appropriate substrate.
One of the biggest issues of today’s medicine is the lack of time. This lack of time leads to nurses being over-solicited, hospitals being overcrowded and doctors being in constant rush. These problems often lead to bad diagnosis, chronic errors in treatment and, ultimately, degradation of the service provided by healthcares.
Indeed, the problem of diagnosis is that almost everything has to be done manually or with different devices that can sometimes be expensive and time-consuming. This diversity of devices is due to the diversity of protocol that has to be performed. With that in mind, we created our device to allow, without losses, the moving of fluids from up to 7 different solutions and samples. It also supports 2 programmable temperature zones and can perform luminometry measurements. Thanks to the EWOD technology allowing fluids to move without losses, we believe that the device could decrease the variability due to multiple manipulators experiments and that it may assure a great repeatability of experiments. With this gain of precision, new fluids could be investigated in the field of biomarkers research, such as tears.
Biosensors are analytical devices that convert a biological response into a detectable signal. The creation of biosensors involves multidisciplinary research in chemistry, biology and engineering. They are applied in many fields, especially in early stage detection of biomarkers because of their enhanced stability and sensitivity as compared with the traditional methods.[14]
By engineering a biosensor, the team thinks that NeuroDrop takes up the challenge of creating an useful application of synthetic biology. This synthetic biological diagnosis tool is firstly appealing because of its flexibility. This characteristic comes from the possibility of changing the aptamer used, making it an opportunity to detect any biomarker from different pathologies.
Synthetic biology allowed the team to have a reliable system, and above all, thanks to the signaling cascade in the bacteria, an amplified signal. This device's important attribute provides the ability to detect small amount of biomarkers that are invisible otherwise without using very advanced and time consuming techniques.
With the increase in life expectancy, more patients are affected by neurodegenerative diseases every day, making them a major health issue for our societies. Among them, Parkinson’s disease (PD) is the second most frequent neurodegenerative disease, right after Alzheimer’s disease [4]. 160 000 patients are currently affected by PD in France and with the 25 000 new cases registered each year, it is estimated that 260 000 patients will be affected in 2030 [15].
Figure 1 Prevalence (a) and Incidence (b) of Parkinson’s disease in France in 2015, sorted by age and sex. Translated from “Epidemiology of Parkinson’s disease, French national data: Frequency of Parkinson’s disease in France in 2015 and trends to 2030”. Frédéric Moisan
Men tend to be 1,5 times more affected by PD than women [15]. The prevalence and incidence both peak (Figure 1) approximately between the age of 85 and 89 years, hence the increasing number of patients affected with the increase of life expectancy. However, it is important to note that both prevalence and incidence seem to be stable overtime (Figure 2). Even if these statistics are centered on France they are corroborated for Europe’s territory in general, as exposed in previous studies [5].
Figure 2 Evolution of prevalence and incidence of Parkinson’s disease in France between 2010 and 2015. Translated from “Epidemiology of Parkinson’s disease, French national data: Frequency of Parkinson’s disease in France in 2015 and trends to 2030”. Frédéric Moisan
The causes of Parkinson’s disease are yet to be discovered. From what we grasp its origin is multi-factorial: combining genetic and environmental factors [4].
Genetic factors: only 15% of PD patients had family history of PD, however 9 genes have been associated with different forms of PD [16]. More gene alterations will probably be discovered in the near future.
Environmental factors statistically associated with PD [16]:
Dopamine is the most abundant catecholamine (a category of neurotransmitters) in the brain. It regulates several physiological functions such as voluntary movements coordination, pituitary hormones secretion etc. [4].
Most of PD symptoms are caused by the progressive degeneration of dopaminergic neurons in the nigrostriatal pathway. A loss of at least 50% of these neurons is necessary before the onset of the cardinal motor symptoms. This degenerative process is not limited to this dopaminergic pathway thus explaining why other motor and non-motor symptoms are resistant to dopaminergic treatment [4].
Parkinson’s disease is a synucleinopathy which means that its pathogenesis is linked to a protein called alpha-synuclein. Neuropathologically, PD is characterized by the presence of proteinaceous inclusions named Lewy bodies which main component is alpha-synuclein. In these inclusions, we find aggregated and fibrillar forms of alpha-synuclein (which pathological aggregation is most likely linked to a C-terminal truncation). The abnormal amount of alpha-synuclein in PD can be linked to its gene (SNCA) which is often duplicated, triplicated and/or mutated in familial forms of PD [17].
Figure 3 Evolution of pathological alphasynuclein. Adapted from “Preventing α- synuclein aggregation: the role of the small heat-shock molecular chaperone proteins” Dezerae Cox Figure 4 Lewy bodies (indicated by arrows) in neurons. Adapted from “Neuropathology: Degenerative diseases” Dimitri P. Agamanolis.
The most-known symptoms for PD are motor: tremor, rigidity and bradykinesia. However, some nonmotor symptoms are to be noted and taken care of: cognitive and behavioral disorders, depression, pain, autonomic dysfunction… [4,6]
We can draw a general outline of the disease progression, after diagnosis, in 4 phases [6]:
As our project was designed to be implemented in France, most of data, health recommendations, diagnostic strategies and treatment are based on the French healthcare system.
In the following explanations, we will focus on the two most represented neurodegenerative diseases: Alzheimer’s and Parkinson’s diseases.
In the vast majority of cases the diagnosis is only clinical, and no complementary exam is justified if the symptoms are typical [4,5]. The diagnosis is assessed by the presence of three cardinal signs and confirmed by the positive response (symptoms relief) to L-DOPA [6].
The three cardinal signs are:
This technique called DaTSCAN is used when the clinical diagnosis is unsure or not possible. It allows the detection by scintigraphy of a functional dopaminergic neuronal termination loss in the striatum [6].
It uses Ioflupane (123I), a radiolabeled neurotracer detected via single-photon emission computed tomography. No serious adverse effects have been registered even though a loss of appetite, headaches and dizziness are sometimes reported by patients undergoing this procedure [7]. Totally reimbursed in France, this test has a 90% sensitivity [6].
No biological diagnosis method is yet available for medical practice. Even though a lot of work is put into the search for new biomarkers, no test has yet proven to be sensitive or specific enough to be of interest over clinical diagnosis and imagery.
Neurochemical biomarkers currently investigated as Parkinson disease (PD) diagnosis biomarkers:
While no curative treatment is available or will be available in the near future, symptomatic treatments currently used have shown a great efficiency in improving patients quality of life. Among those, 3 main categories of pharmacological treatment are used [18,19]:
Figure 5 French recommendations of treatment choice presented as a decision tree. Adapted from “Vidal Recos” 2019.[18]
A medical procedure used as a PD treatment, originally developed here in Grenoble has proven astonishing efficiency: Deep Brain Stimulation (DBS). Very few patients are treated by this method as it is only recommended in very specific Parkinson’s disease configuration.
New strategies targeting alpha-synuclein expression are currently flourishing. Magnetic nanoparticles able to be delivered through the Blood-brain Barrier to target dopaminergic neurons have been engineered. Gold nanoparticles used to load plasmid DNA in specific neurons have demonstrated to be able to suppress alpha-synuclein overexpression and even to inhibit dopaminergic neuron apoptosis [20]. Alpha-synuclein immunotherapy, gut targeting, alpha-synuclein receptor inhibition etc. are also being investigated by diverse research teams [17].
 NeuroDrop
NeuroDrop
Early detection for patient care improvement:
challenges of today’s diagnosis toolsEARLY DIAGNOSIS: IMPORTANT PUBLIC HEALTH STRATEGY [1]
DIFFICULT DIAGNOSIS AND CONSEQUENCES
Highlighting the medical potential of tears:
our NeuroDrop biosensorBENEFITS OF THE TEAR FLUID


The utility of such a versatile biosensor could also be multiplied by another factor: time. Because we designed an automated system of detection, only few steps are needed, thus allowing people to win a lot of time, an eternally rare resource. In addition, this accessible liquid can be sampled without high expertise, only a short and simple formation is required.
HOW DOES IT WORK?

The EWOD plate is used to move the small drops of tear fluid, NeuroDrop bacterium and reagents needed in the system.
Contacting the fuilds will lead to a bioluminescence signal produced by the bacteria that can be measured. 
APTAMERS
For our proof of concept, Aptamer-based sandwich-type biosensor was made with two aptamers recognizing two epitopes of the protein of interest: the oligomeric alpha-synuclein.
More informations on the design page.
Clickable Outer Membrane Protein (COMP)
Previously, the aptamer has been fused with a cyclooctyne chemical group, interacting with the azide group (azido phenylalanin) to perform a cycloaddition without copper catalysis (lethal for bacteria).
More informations on the design page.


Outer membrane Bacterial Adenylate Cyclase Two Hybrid (mBACTH)
The recognition of the protein of interest by aptamers at the surface of the bacteria allows COMP proteins to get closer. This precise spatial change enable the AC to regain its native conformation and physiological activity (i.e. cAMP production from ATP).
More informations on the design page.
NanoLuciferase reporter gene
More informations on the design page.
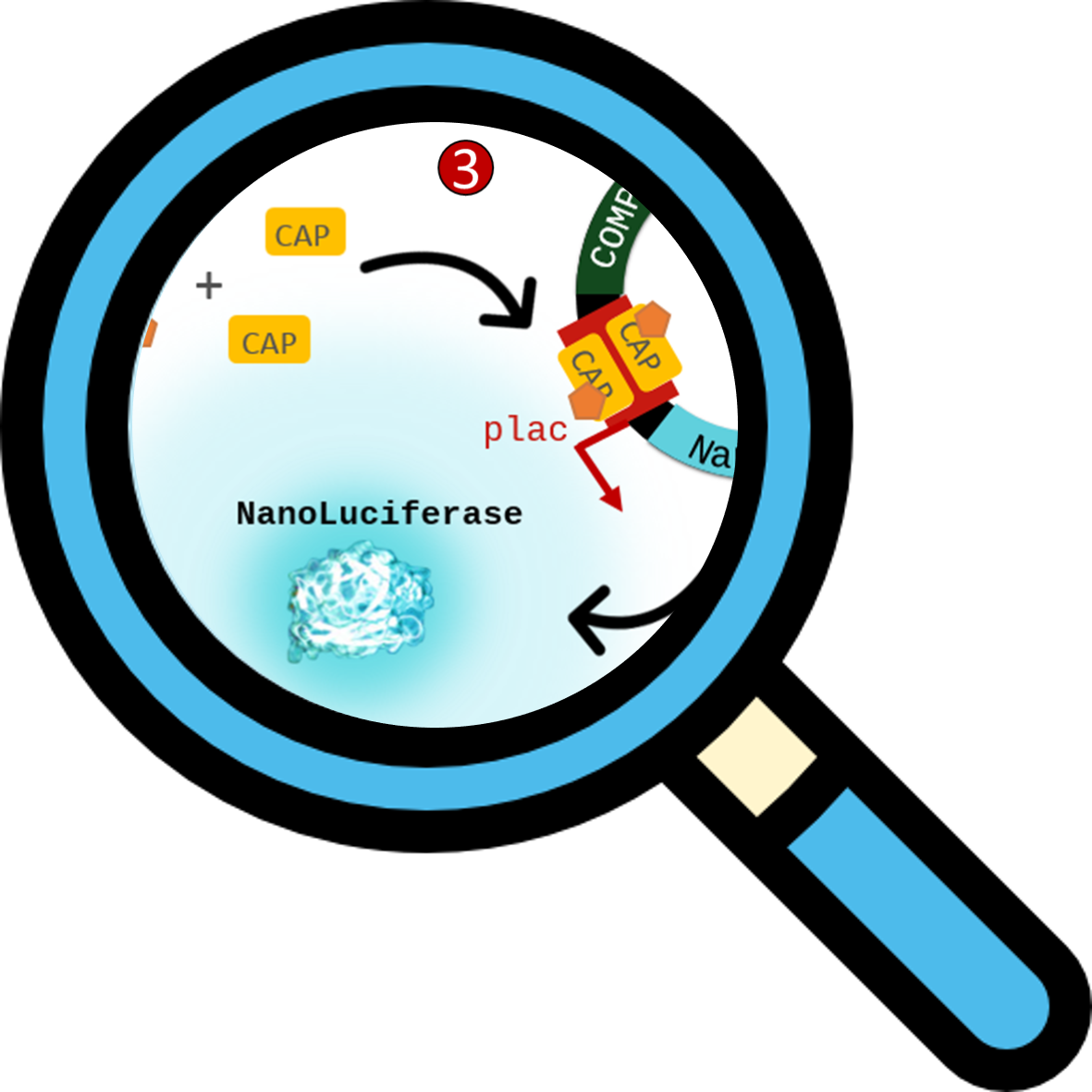
A NEW WAY TO BACTH
In this attempt, a genetically modified Clickable Outer Membrane Protein OmpX named COMP is expressed at the bacterial cell surface on which specific aptamers are clicked, thus generating a COMB (Clickable Outer Membrane Biosensor). The click reaction is instantaneous, and it can be performed in physiological conditions. Therefore, specific aptamer can be easily switched to detect a particular biomarker of interest. Thus the huge biological flexibility is one of the main benefits of our system. Our COMB is able to detect every desired biomarker as long as the appropriate specific aptamer sequence is available.
The system works by complementation of the two sub-parts of the AC. In absence of biomarker in the environment, T18 and T25 COMBs move freely in the external membrane, so both sub-parts are distant and do not have an enzymatic activity. In contrast, when a biomarker is recognized, the COMBs and so the two subparts, are brought together to reconstitute the AC which recover an enzymatic activity.
BENEFITS OF AN ELECTRO-WETTING-BASED DEVICE
To address this issue, we propose an automated system enabling a simultaneous multi-samples analysis using small volumes.
Benefits of a synthetic biology detection tool
Another flexibility asset of NeuroDrop is that, in addition to tears, the system allows the utilization of different non complex fluids such as saliva, sweat or urine. Thanks to its capability to use smaller volumes than other current diagnosis tools. The automation is amazing, because several detections can be made at the same time.
Moreover, the price and the easy utilisation of such a tool are two main positive aspects of the device. Also, synthetic biology guarantees the possibility to adjust the system and to do plenty of modifications.
Proof of concept: Parkinson's disease
diagnostic strategies and treatment are based on the French healthcare system.
EPIDEMIOLOGY


PHYSIOPATHOLOGY
CAUSES
PATHOGENESIS


PROGRESSION AND SYMPTOMS
DIAGNOSIS
CLINICAL DIAGNOSIS
IMAGERY DIAGNOSIS

BIOLOGICAL DIAGNOSIS
TREATMENTS

References
*BACTH: Bacterial Adenylate Cyclase Two Hybrid.
As our project was designed to be implemented in France, most of data, health recommendations,
