Introduction
Escherichia coli Nissle 1917 (EcN) is probably the most intensively investigated bacterial strain today [1]. Despite the fact that the EcN strain is widely used as a probiotic, a lot of questions remain. Hence, we decided to dive deep into the characterization of EcN. Our goal was to find out more about EcN itself and to provide crucial information to include EcN as a platform organism for other iGEM Teams and researchers.
Growth Curves
The first step in the characterization of a bacterium is conducting growth experiments in different media and under different conditions. This helps to get a better understanding of beneficial conditions for growth of the culture, which will improve folliwing experiments. We chose to perform half of our experiments under aerobic and the other half under anaerobic conditions. As a control, we grew EcN in LB medium at 37°C and pH 7 under aerobic conditions. We tested growth at a variety of different temperatures (25°C, 8°C, 4°C and 0°C) showing that EcN growth is inhibited at 8°C, the temperature often used for cold shock (Fig. 1).

Moreover, we evaluated the growth in the pH range from pH 6 to pH 1, since in our application EcN will have to pass the acidity of the stomach. Here, at pH 4 we discovered an interesting recovery of EcN growth after three hours (Fig. 2).

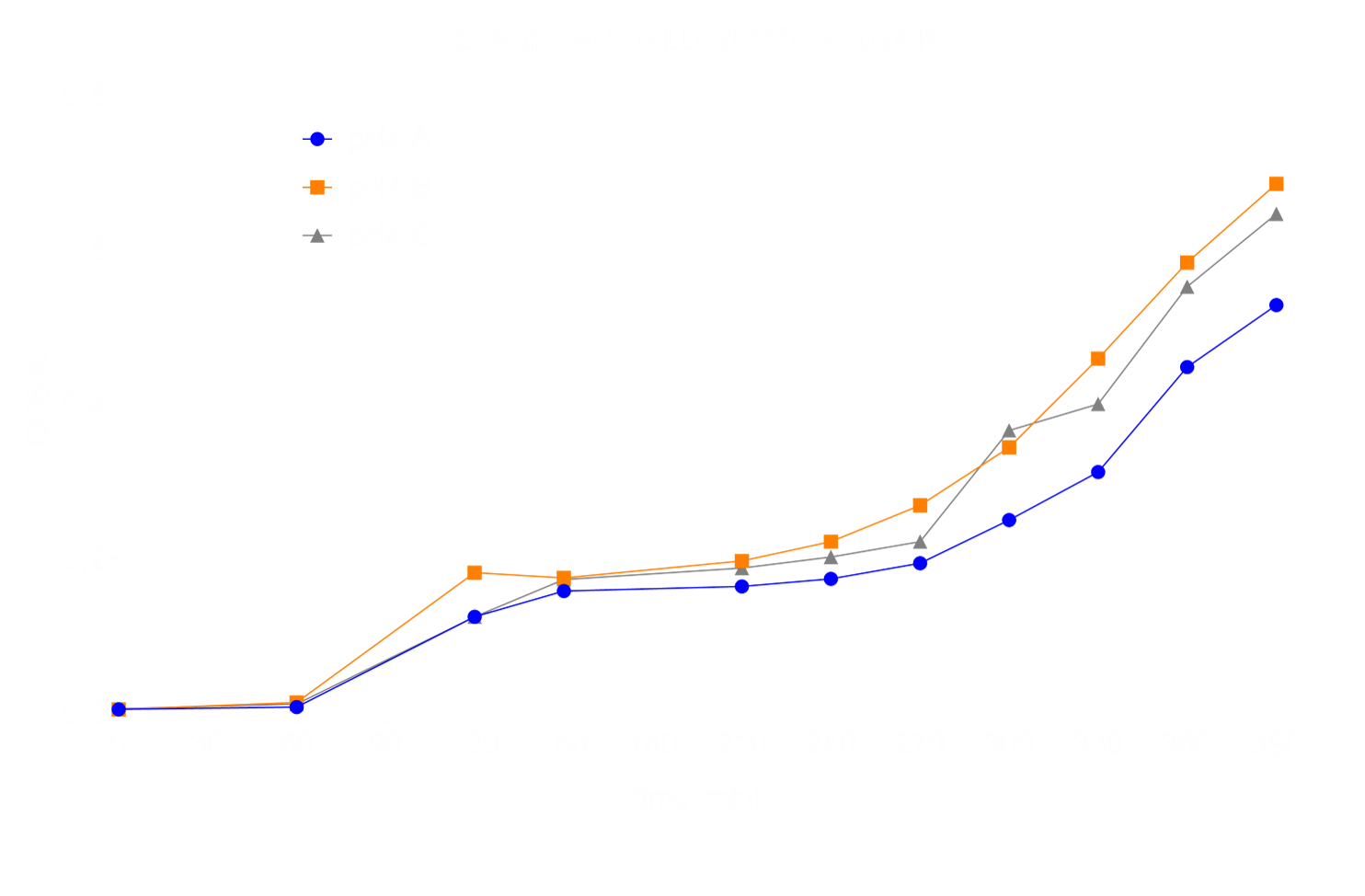
To look into inflammatory stress, we subjected EcN to up to 100 µM hydrogen peroxide [2] and showed that the growth is not substantially influenced by this chemical (Fig. 4).

Lastly, we performed dryfreezing and recovered EcN afterwards under control conditions [3][4][5]. The experiment showed that dryfreezing within skim milk protects the bacteria from substantial death (Fig. 5).

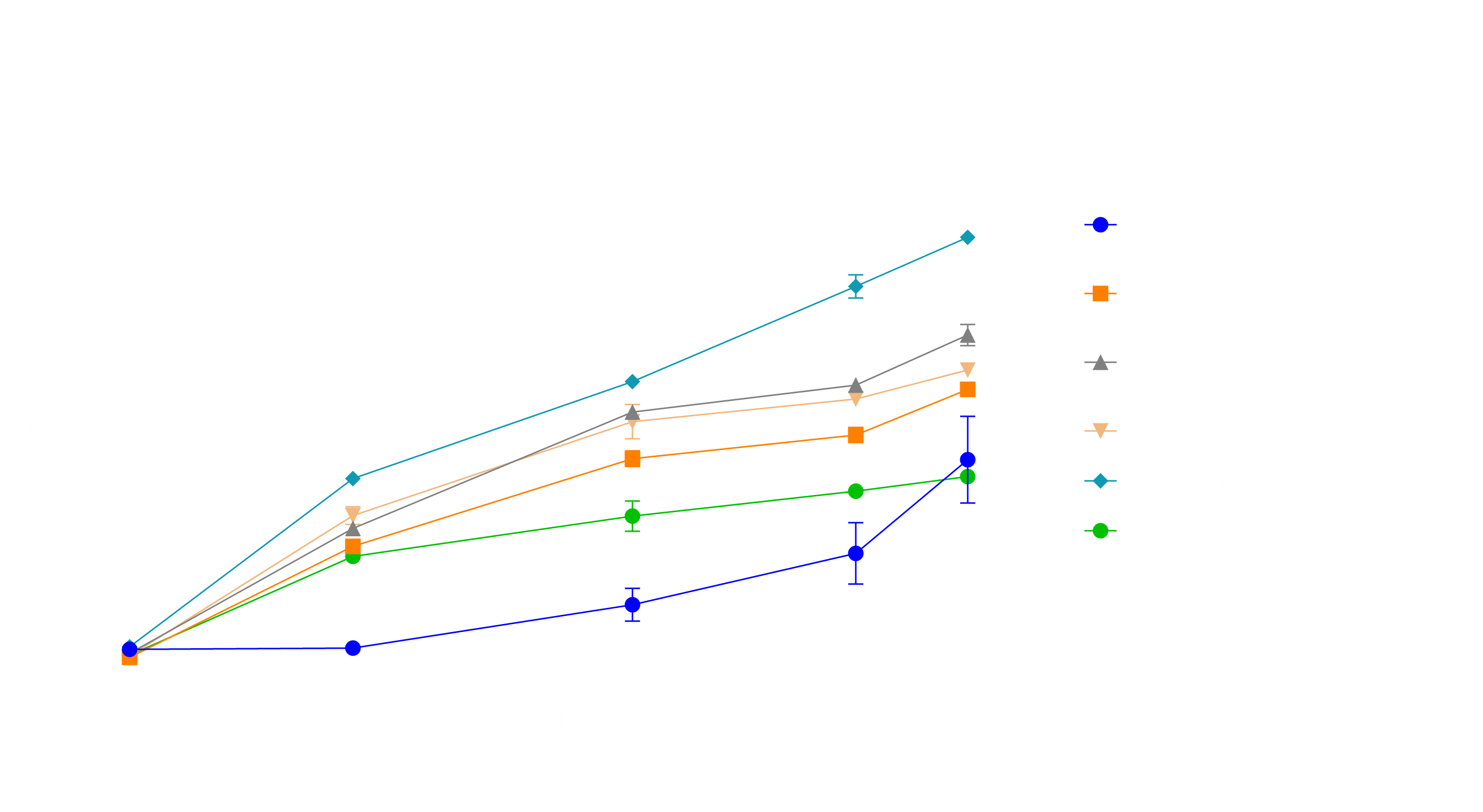
Anaerobic conditions were used to characterize EcN growth under the circumstances provided in the human intestines. Thus, as a control we compared EcN growth in LB medium versus mGAM medium under anaerobic conditions. The experiment showed that mGAM, a medium designed for anaerobic application, is in fact a more suitable medium (Fig. 6). Then, we subjected the culture to metformin treatment, since it is one of the most common treatments of Diabetes and is known to accumulate to 720 µM to 7.2 mM within the intestines due to low bioavailability [6][7]. We showed that EcN growth is not substantially influenced by 1.3 mM of metformin into mGAM medium (Fig. 6). Moreover, we tested for EcN’s resistance against cholic acid, since it is commonly secreted into the ileum. Here, we added 0.25 mM cholic acid to mGAM medium, considering that the concentration of free bile salts may rise to 0.25 mM to 1 mM of total bile salts in the Ileum, with cholic acid only being one component [8][9]. Our results suggested that EcN growth is not greatly influenced by cholic acid (Fig. 6).

Finally, we tested EcN interaction with other bacteria by adding bacterial supernatant to our medium. The supernatant was sterilized and provided by Dr. Lisa Maier. We chose Bacteroides thetaiotaomicron, Prevotella copri and Ruminococcus gnavus since they are commonly found within the human microbiome [10]. Bifidobacterium adolescentis was chosen due to its probiotic nature and Clostridium difficile supernatant was used, since it is often found in the microbiome of people with chronic inflammation [10][11]. The results suggest that EcN grows under all additions, however was initially inhibited in its growth by the addition of Bacteroides spp., thus we used these samples for RNA-Seq (Fig. 7).
RNASeq
The transcriptome describes the set of all RNA molecules in a population of cells and is subject to continuous changes. Understanding the complete transcriptome, the expressed genes, post-transcriptional modifications and additional properties of interest is imperative towards understanding genetic cause, disease and possible treatment strategies.
The inherent complexity of the transcriptome requires precise and scaling analysis techniques. RNA sequencing (RNA-Seq), also known as whole transcriptome shotgun sequencing (WTSS), is most commonly used for this purpose, today. It uses next-generation sequencing (NGS) to detect and quantify RNA in biological samples.
The generated RNA-Seq read data is then analyzed according to a sample RNA-Seq work-flow (Fig. 7).
Next, a statistical analysis yields all differentially expressed genes, which are used for Gene Ontology (GO) enrichment, as well as pathway analysis.
We set ourselves the goal of understanding the transcriptomic changes that EcN undergoes under various stress conditions to gain a deeper insight into its responses. Understanding these stress responses of E. coli Nissle 1917 could lead to the development of more robust strains, which not only our project would benefit from, but also scientists working on probiotic drugs in general.
We divided our experimental design into two parts. One part was to investigate the effect of environmental stress on E. coli Nissle 1917 under aerobic conditions, the other part was to examine the responses under anaerobic conditions. Hence, our experimental design looks as follows:
| Aerobic | Anaerobic |
|---|---|
| LB, pH 7, 37°C | LB, pH7, 37°C |
| LB, pH7, 25°C | mGAM, pH7, 37°C |
| LB, pH7, 8°C | mGAM + Metformin, pH7, 37°C |
| LB, pH4, 37°C | mGAM + Cholic Acid, pH7, 37°C |
| LB, 100µM H2O2, 37°C | mGAM + Bacteroides SN, pH7, 37°C |
| LB, 37°C post dry-freeze, pH7 | Control, aerobic, LB, pH7, 37°C |
The respective temperature, pH values, and doses were determined by growth curves (Figures 1-6). To get the most out of RNA-Seq applied to stress factors, it is important to find the cutoff values, where E. coli Nissle 1917 is put under stress the most, but still survives.
Sequencing and library preparation was conducted on two sites. All aerobic samples were prepared and sequenced at the NGS Competence Center Tübingen (NCCT), whereas all anaerobic samples were prepared and sequenced at the European Molecular Biology Laboratory (EMBL) in Heidelberg. For more details please visit our Notebook and Outlook.
The following data analysis was, therefore solely conducted on the EMBL dataset.
Data Analysis
Since we received our sequencing data from the NCCT on the 18.10.2019, we weren’t able to analyze the data according to our standards in this short time frame. However, as noted in our Attributions, we will analyze both datasets in depth and are planning to publish the results next year
Workflow
Our complex and cutting-edge data analysis is based on several workflows and tools. The initial quality control and feature counting was conducted using the highly sophisticated nf-core/rnaseq pipeline. The pipeline is implemented in nextflow and runs singularity containers to absolutely ensure reproducibility. The workflow processes raw data from FastQ inputs (FastQC, Trim Galore!), aligns the reads (STAR), generates counts relative to genes (featureCounts) or transcripts (Salmon, tximport), and performs extensive quality-control on the results (RSeQC, Qualimap, dupRadar, PreseqPreseq, edgeR, MultiQC).
The differential gene expression analysis was conducted using qbic/rnadeseq, a nextflow based pipeline, which was also run with a singularity container for reproducibilities sake. The workflow runs DESeq2 for the detection of differentially expressed genes. The pathway analysis was conducted manually using clusterprofiler.
Quality Control
The following is only a small selection of all quality control metrics. A focus is set on the most interesting and important quality control metrics. We highly recommend visiting our downloads page to download the full interactive multiqc report. It features short explanations of the quality metrics and lots of useful plots.

The quality of the reads at every position has a Phred score of at least 32, demonstrating that the sequencing quality is very high (Figure 8). Moreover, the usually observed decline in quality at the end of the reads due to phasing is exceptionally small.

Duplicated reads were assessed using DupRadar. DupRadar provides duplication rate quality control for RNA-Seq datasets. Highly expressed genes can be expected to have a lot of duplicate reads, but high numbers of duplicates at low read counts can indicate low library complexity with technical duplication. Our distribution follows the expected trends. Hence, we can rule out excessive rRNA contamination or excessive PCR artifacts.
To verify that our replicates are indeed replicates of the same condition, and also to explore whether or not our various conditions indeed have a biological effect and differ from the anaerobic ground truth, we created a Multidimensional scaling (MDS) plot using edgeR. MDS visualizes the level of similarity of individual cases of a dataset.
It is used to translate "information about the pairwise 'distances' among a set of n objects or individuals" into a configuration of n points mapped into an abstract Cartesian space. MDS is a form of non-linear dimensionality reduction.

It can be observed, that the majority of the reads are protein coding, with some mapping to sRNA and rRNA. Sample four shows significant rRNA contamination, but still in tolerable numbers.
All in all, our extensive quality control suggests that the libraries were well prepared and rRNA or adapter contamination is very low with anaerob sample four posing a small exception. However, since the quality was high enough according to our standards, it was still included in all downstream analyses. Our initial quality control already suggests, that biological differences between the samples are very likely to exist. These will be examined more in depth in the first step of our downstream analysis: Differential expression analysis.
Differential Expression Analysis
Pathway Analysis
Metabolic Model
Our E. coli Nissle 1917 characterization includes our metabolic modeling. Please visit our model subpage for more details.
Metabolic Model
Insert here
References
- Ulrich Sonnenborn, Escherichia coli strain Nissle 1917—from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties, FEMS Microbiology Letters, Volume 363, Issue 19, October 2016, fnw212,
- Sana Ben Othman and Tomio Yabe. Use of Hydrogen Peroxide and Peroxl Radicals to induce Oxidative Stress in Neuronal Cells. Reviews in Agricultural Science, 3:40-45, 2015. Doi: 10.7831/ras.3.40
- P. Capela, T.K.C. Hay, N.P. Shah. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yoghurt. Food Research International. Volume 39, Issue 2, 2006, Pages 203-211, ISSN 0963-9969, https://doi.org/10.1016/j.foodres.2005.07.007.
- Govender M, Choonara YE, Kumar P, du Toit LC, van Vuuren S, Pillay V. A review of the advancements in probiotic delivery: Conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS PharmSciTech. 2014;15(1):29–43. doi:10.1208/s12249-013-0027-1
- https://opsdiagnostics.com/applications/lyophilization/ecoli_lyophilization_stability.html
- Bailey, C. J, Wilcock, C, Scarpello, J. H. B. Metformin and the intestine. Diabetologia. 2008. Volume 51, Issue 8, Pages 1552, ISSN 1432-0428, Doi: 10.1007/s00125-008-1053-5,
- High Accumulation of Metformin in Colonic Tissue of Subjects With Diabetes or the Metabolic Syndrome. Paleari, LauraBurhenne, JürgenFoersch, SebastianParodi, AndreaGnant, MichaelScherer, DominiqueUlrich, Cornelia M.Stabuc, BorutPuntoni, MatteoCoccia, GianniPetrera, MarilenaHaefeli, Walter-EmilDeCensi, Andrea et al. Gastroenterology, Volume 154, Issue 5, 1543 - 1545
- Martinez-Augustin O, Sanchez de Medina F. Intestinal bile acid physiology and pathophysiology. World J Gastroenterol. 2008;14(37):5630–5640. doi:10.3748/wjg.14.5630
- Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. T. C. NORTHFIELD AND I. McCOLL. Gut, 1973, 14, 513-518
- Belizário JE, Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 2015;6:1050. Published 2015 Oct 6. doi:10.3389/fmicb.2015.01050
- Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8(1):51. Published 2016 Apr 27. doi:10.1186/s13073-016-0307-y































