Results
Preparation
Competent cell preparation
For DH10B, the number of colonies was calculated and the transformation efficiency of the DH10B strain was around 5.23E+3.

Vector DNA preparation
The extraction concentration of each vector is as follows; pB1: 27.087ng/㎕, pB2: 27.201ng/㎕, pB3: 21.234ng/㎕, pB4: 23.218ng/㎕, pB5: 21.298ng/㎕, pB6: 19.266ng/㎕, pB7: 21.398ng/㎕. Electrophoresis results show a single band on all vectors.
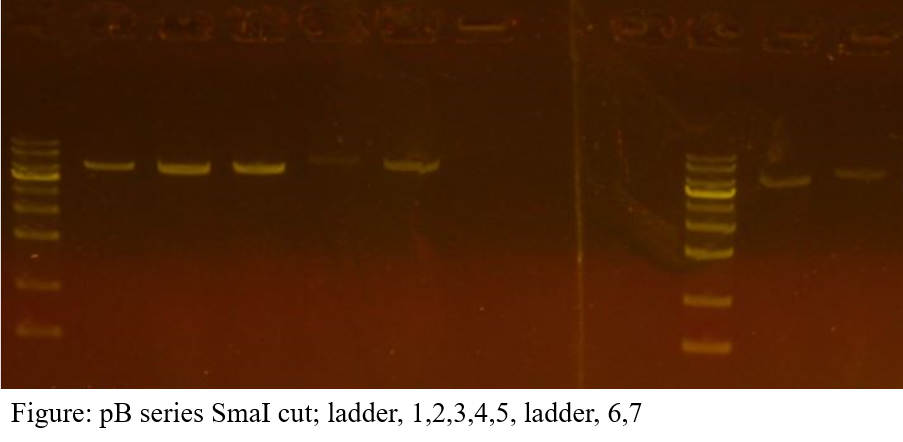
DNA work
Transformation
Clones for several pB vectors were obtained. The picture below shows the results of successful cloning of pB3 and pB7 vectors. Although there is only one picture, we repeated this process several times, eventually adopting a cloned strain using pB3 and pB4 vectors.

Minipreparation
In the overall cloning process, about two failures were experienced, resulting in the acquisition of luc2-pB3 and luc2-pB4, hydroxy-pB3 and hydroxy-pB4 plasmid.
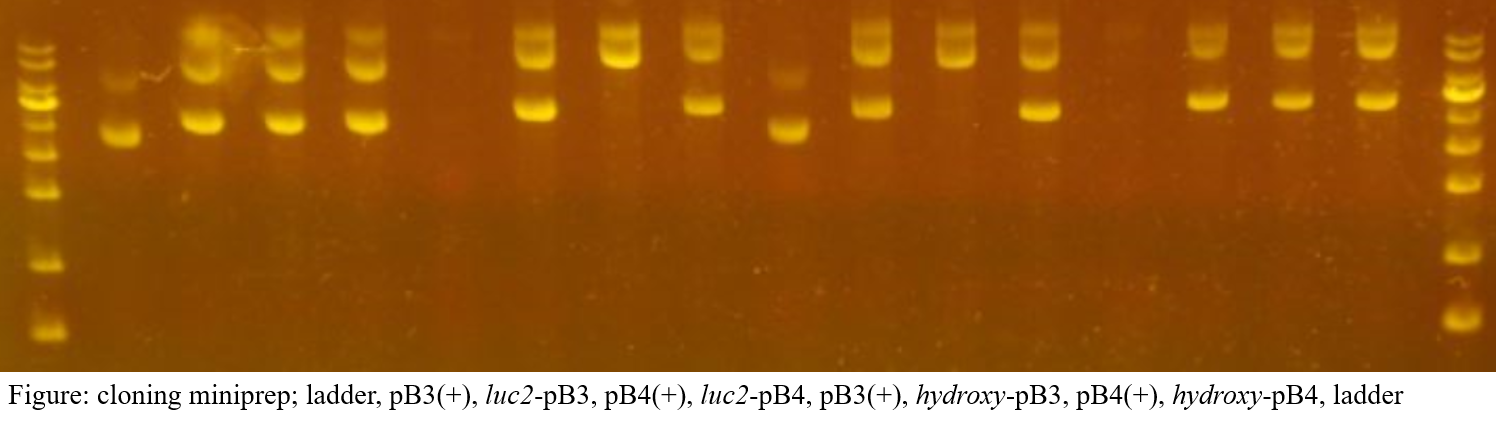
Protein work
Expression test
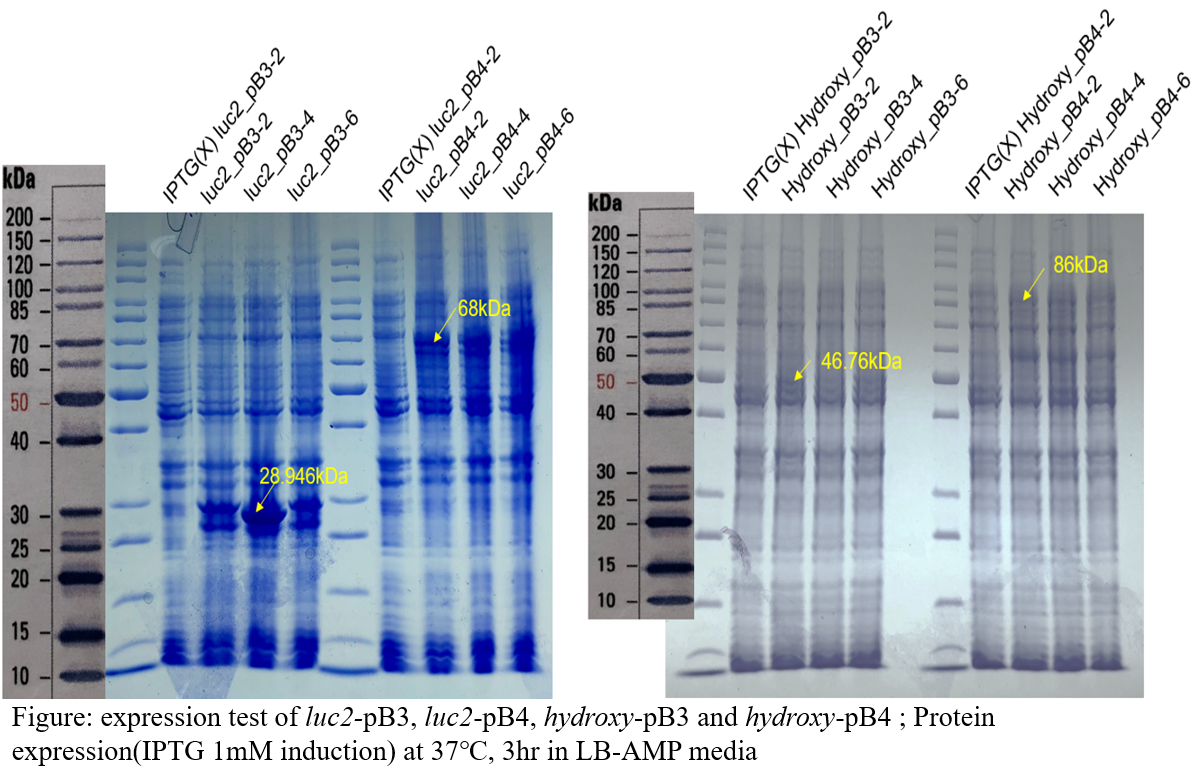
As a result of SDS-PAGE, the bands were identified in both the luc2 and hydroxy genes’ recombinants, so the desired protein was expressed in the host. The different bands have different heights depending on the type of vector, because different tags are linked to the protein. The tag of the pB3 vector has a size of 3,128Da and the tag of pB4 vector is 43,623Da.
Solubility test
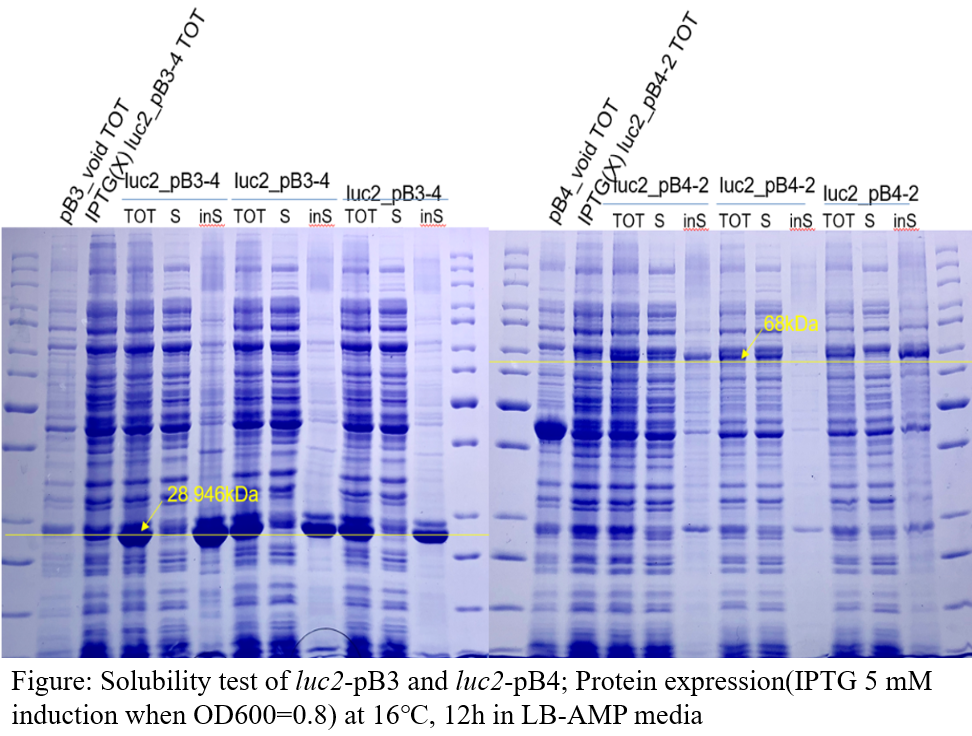
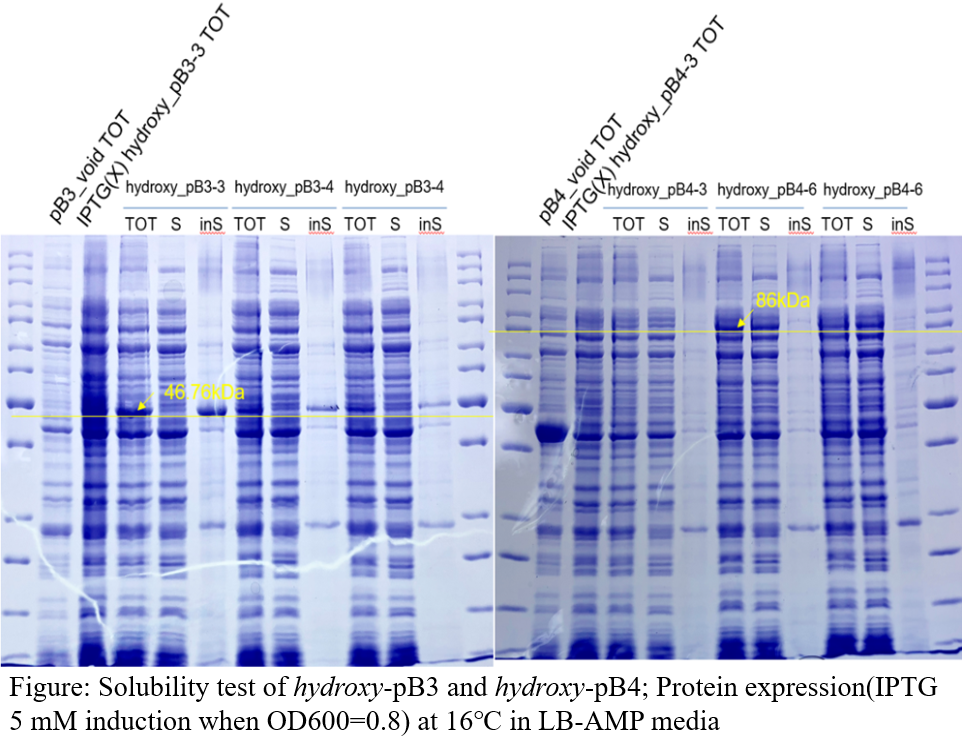
Test’s result shows that only genes assembled into the pB4 plasmid showed protein in the soluble part. Therefore, we decided that subsequent identification of activity would be carried out using only this plasmid.
Activity test
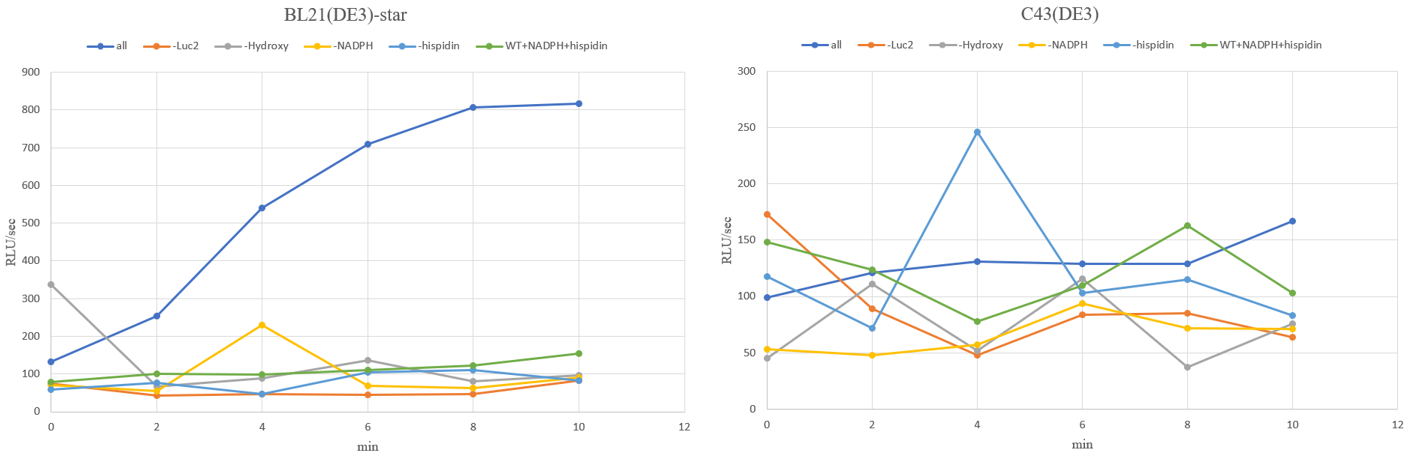
The results of observing the luminous flux of the experimental group with the control group for 10 minutes are as follows for each strain.
The luminous flux of O.japonicus with the addition of hispidin was also measured, and the results are as follows.
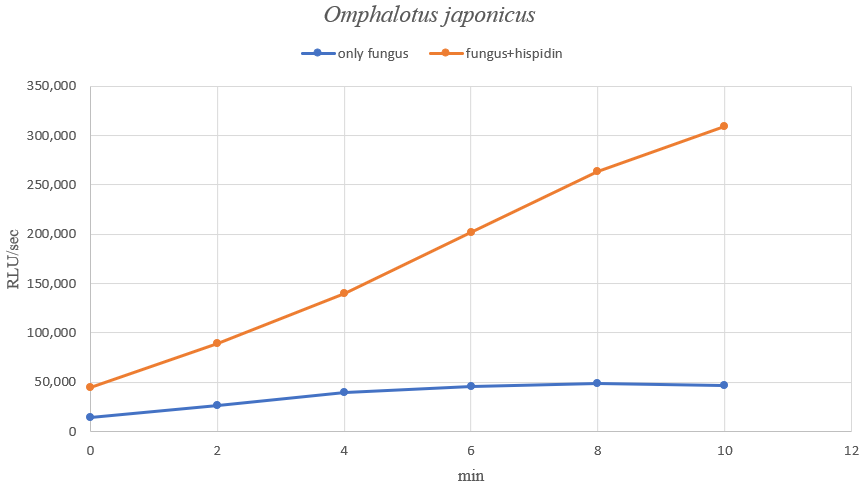
This confirmed that hispidin works as a fungal luminescent element. We can therefore see the activity of the fungal luminous enzyme in the test tube. However, compared with the amount of light emitted in mushrooms, it seems that in vitro emits are quite low levels. It can be reasonable to think that the activation factor for luminescence may additionally act in vivo, or the results may vary depending on the proportion of necessary components. In other words, this result show the possibility that our system can produce strong enough light through optimization tasks.
The results of the measurement of luminous flux for an additional 12 hours(5-minute interval) are as follows.
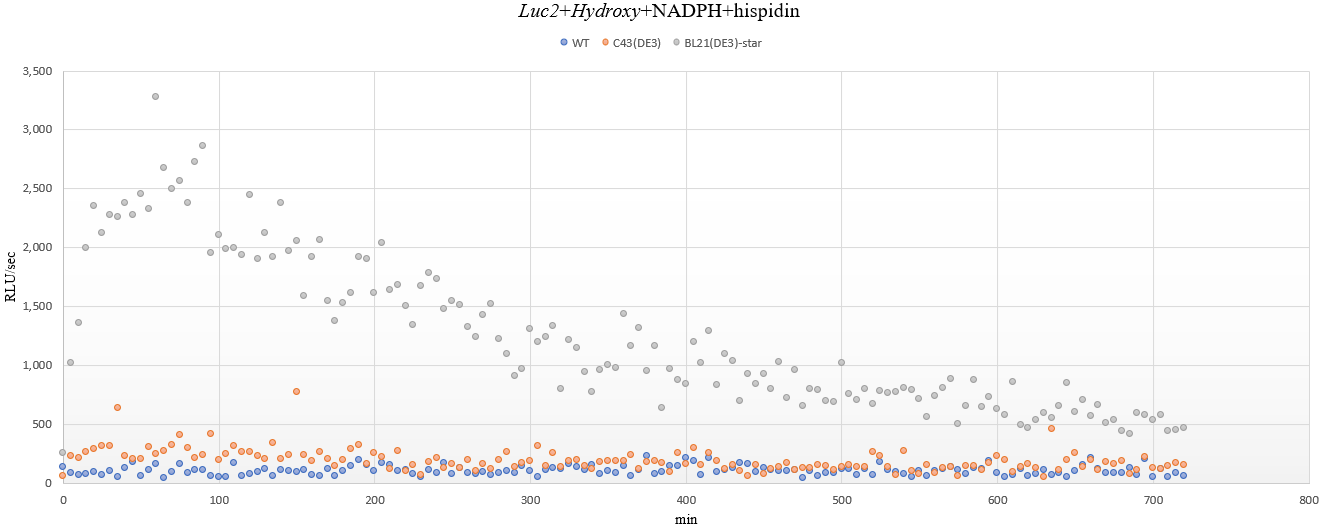
From the above results, the luminous flux graphs for each strain are shown below, which subtracts the mean of background noise represented by wild type, C43(DE3).
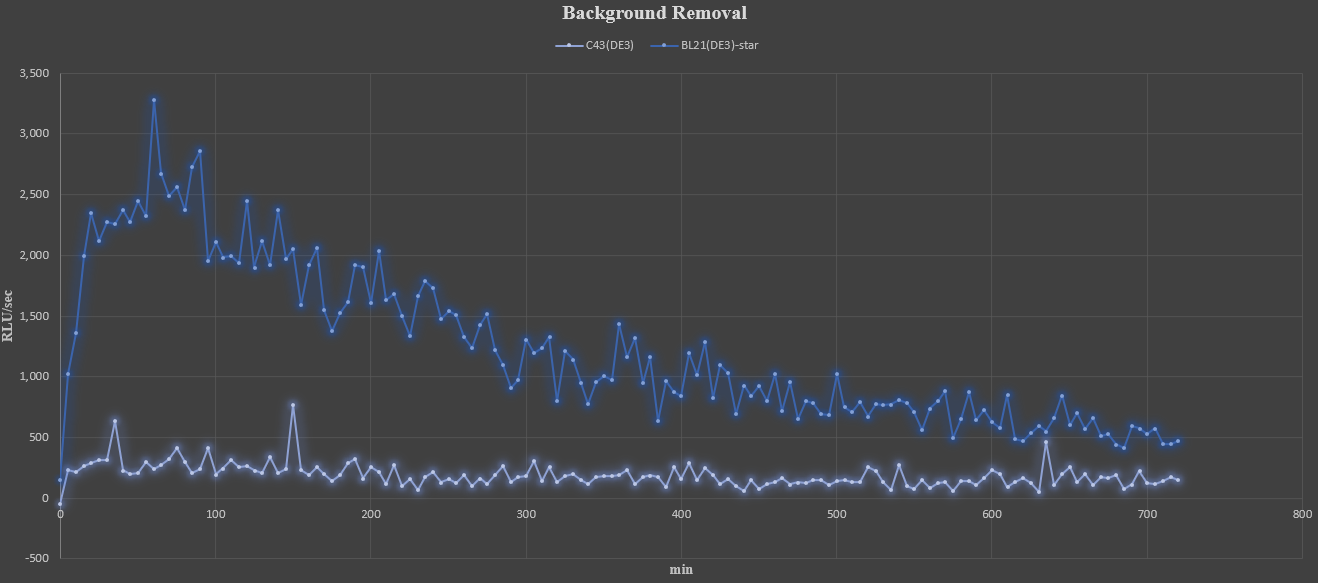
At the beginning of measurement, it can be seen the luminous flux gradually increases and then decreases slowly after reaching its peak. In addition, the early experiments of 10 minutes-measurement were not sure, but a small amount of luminescence was still observed in the C43(DE3) strain. Although C43(DE3) is uncertain because it has not been tested for solubility, it can be expected that most enzymes have been converted into insoluble proteins in the process of over-expression.
