Design
As mentioned earlier, the goal of our project is to develop bioluminescent tattoo that shows the degree of stress.
To produce this, we had to set up a substance that our synthetic biological system would detect as an indicator of stress, which is cortisol.
There are two main reasons for choosing Cortisol as an indicator of stress.
1. It is used as an indicator of stress in various studies and as a bio-indicator in many tests.
2. It acts as a stress hormone in most vertebrates such as pigs and fish as well as humans.
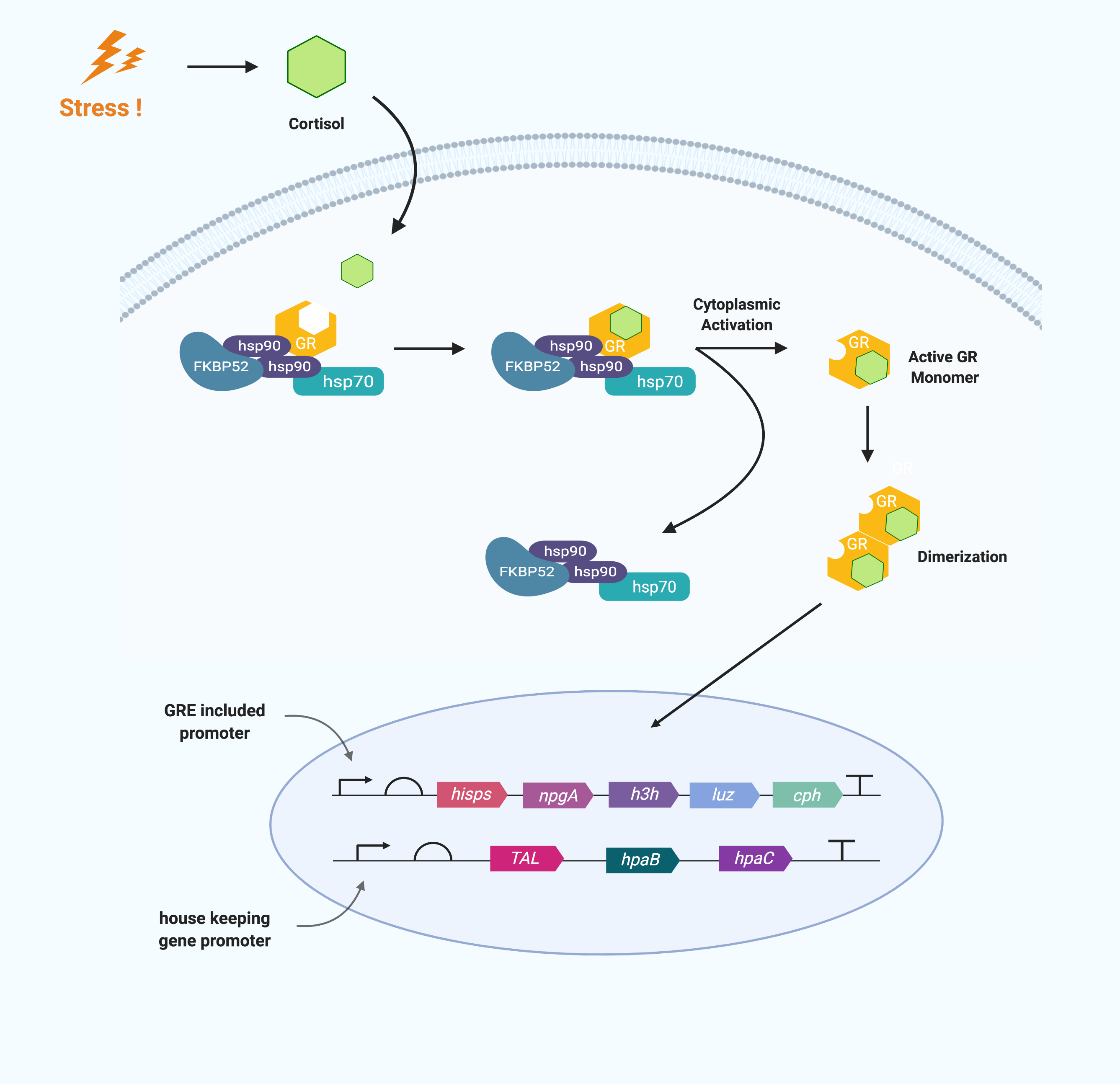
Cortisol is a glucocorticoid that is fat soluble as a steroid hormone. Thus it can pass directly through the phospholipid bilayer without membrane protein. After passing through the cell membrane, cortisol binds to the Glucocorticoid receptor in the cytoplasm. Glucocorticoid receptors (GR) are cytoplasmic in the absence of ligand and complex with chaperones. When cortisol enters the cell, it binds to GR and dissociates chaperones from GR. Then, it exposes hidden nuclear localization signals forming a cortisol–glucocorticoid receptor complex. This cortisol–glucocorticoid receptor complex homodimerizes and migrates to nucleus. The homodimer binds with high-affinity to DNA binding sites (DNA sequences) called glucocorticoid-responsive elements (GRE), which are present in the promoters to activate or inhibit transcription.
The mechanism of GR varies according to the type of GRE.
The “simple” GRE is an imperfect palindromic sequence consisting of three base pairs and two hexameric half sites on either side, and GR binds directly to regulate transcription. In particular, the GRs that bind to it mediate enhancing transcription rather than repressing.
The "composite" GRE has a binding site where the GR and the transcription factor bind together. The “Tethering” GRE participates in the way that GR regulates the target gene indirectly. It does not have a DNA binding site for GR but has a binding site for another transcription factor that binds to and works with GR.
The group of GRE contains a positive GRE that promotes transcription and a negative GRE that suppresses it, and we plan to use a promotor that includes a positive GRE.
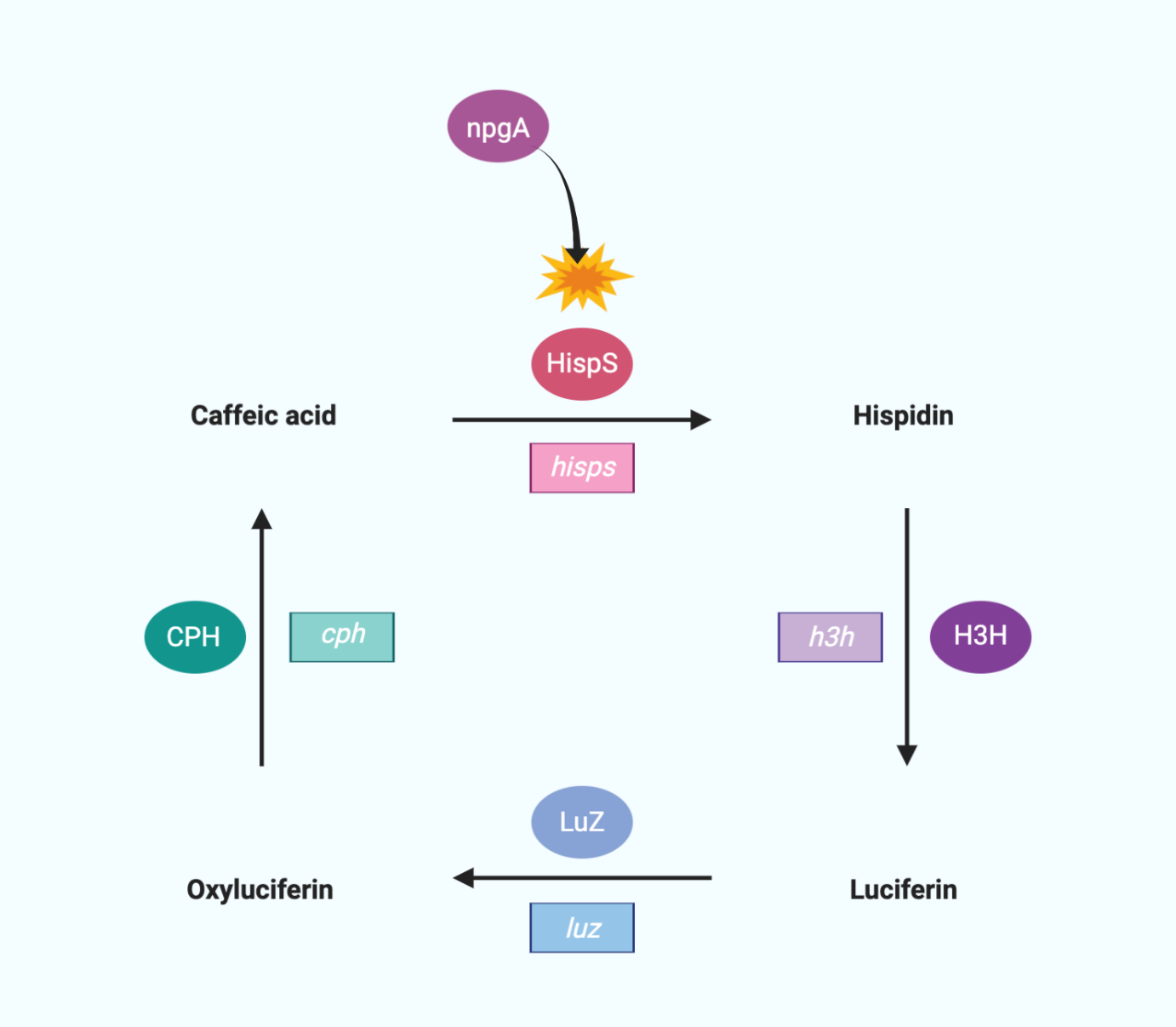
We use a bioluminescent circuit that starts with caffeic acid.
HispS(hispidin synthase) makes Caffeic acid hispidin, and H3H(hispidin 3-hydroxylase) makes hispidin 3-Hydroxyhispiidin. 3-Hydroxyhispiidin is used as luciferase in this circuit. When Luz makes 3-Hydroxyhispiidin a high energy intermediate, it emits light and becomes caffeylpyruvic acid (oxyluciferin). The CPH(caffeylpyruvate hydrolase) then turns it into caffeic acid, which completes the cycle. In addition, since HispS is a member of the polyketide synthase family, the large modular polyketide synthases require posttranslational modifications for their activity. Therefore, we added the npgA gene which encodes 4′-phosphopantetheinyl transferase originating from Aspergillus nidulans for hisps gene activity.
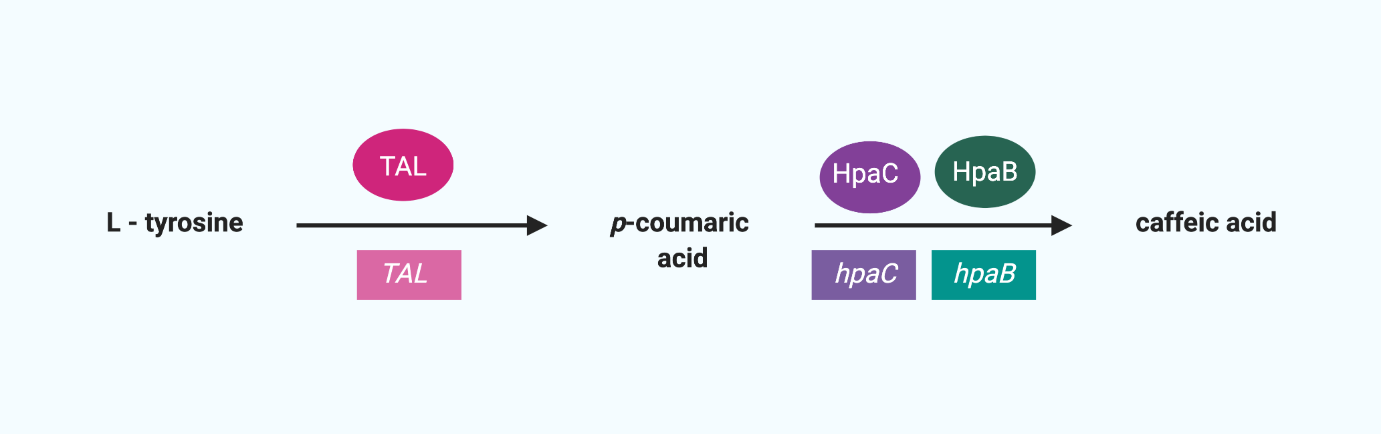
Also, the fungal bioluminescent system we are going to use requires caffeic acid, so we need to make caffeic acid in the cells. Therefore, we added a gene circuit to synthesize caffeic acid.
One of the essential human amino acids Tyrosine (Tyr) is converted to p-coumaric acid using enzymes made by TAL, and p-coumaric acid is converted to caffeic acid using enzymes made by hpaC and hpaB.
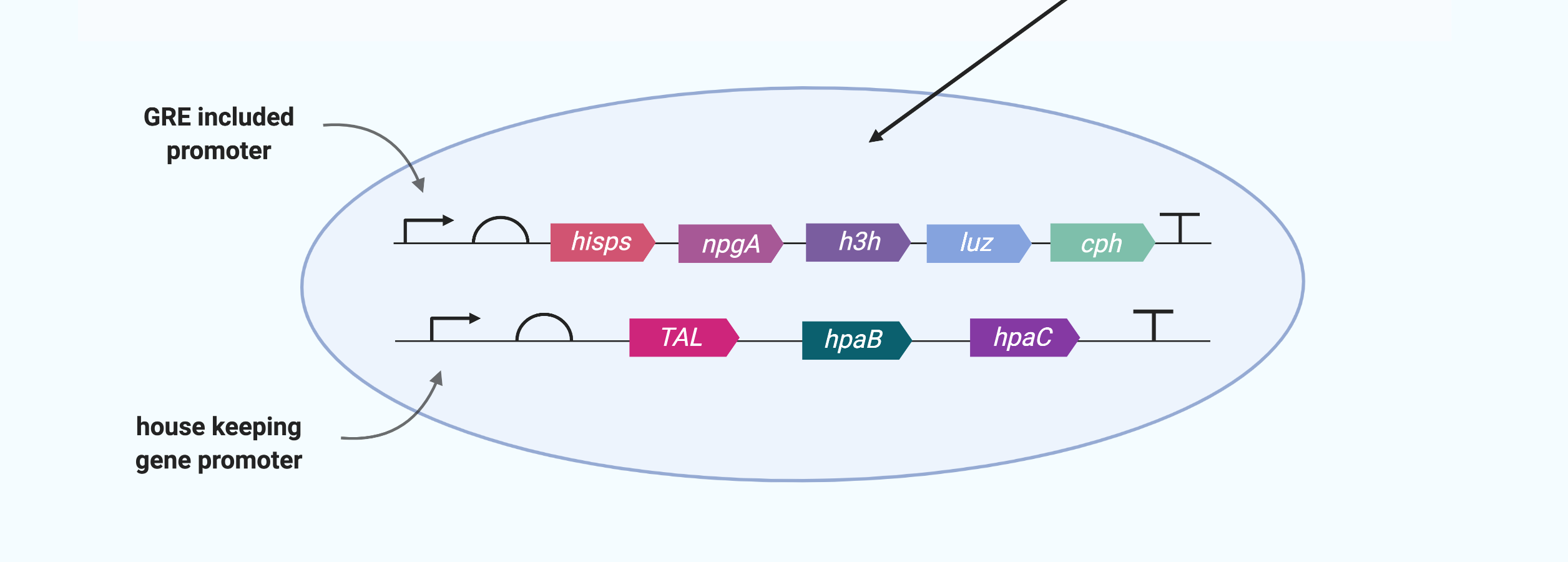
After constructing the gene circuits as above, next step is transfecting the cells to be used with the them.
Prior to the transfection step, the promoter to be used for each gene circuit must be determined. The caffeic acid production gene circuit uses Tyrosine, an essential amino acid, so it is advantageous for the cell to work only in stress situations. Therefore, we use a promoter with GRE in this circuit. Bioluminescent circuits, on the other hand, should work as soon as a large amount of caffeic acid is produced, so relevant enzymes must always be present in the cell. Therefore, we use a house-keeping gene promoter that would operate constantly in bioluminescent gene circuits.
As a transfection method, we will use 'stable transfection', where the target sequence is inserted into the chromosomal DNA in the target cell, which can produce a longer-term effect.
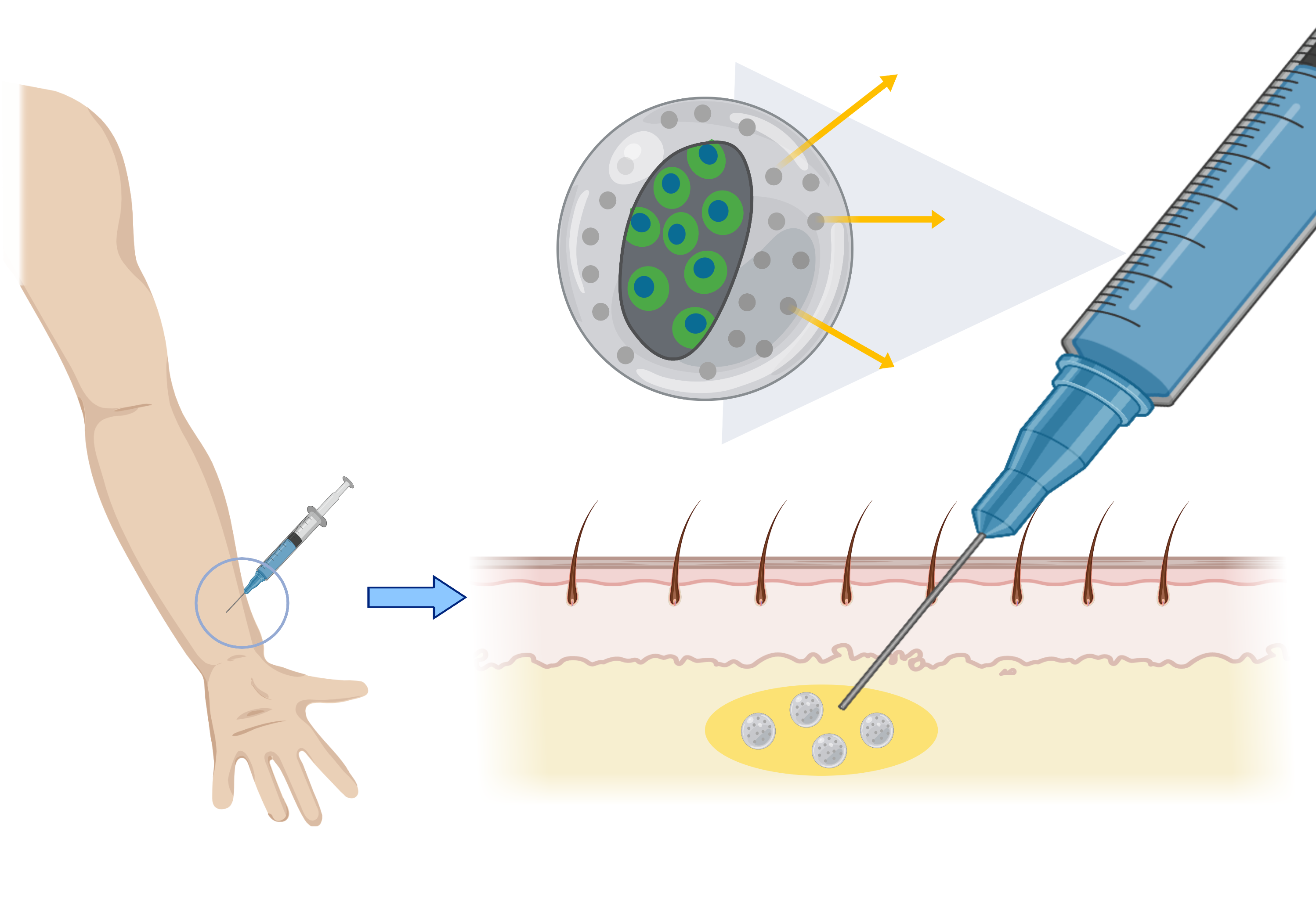
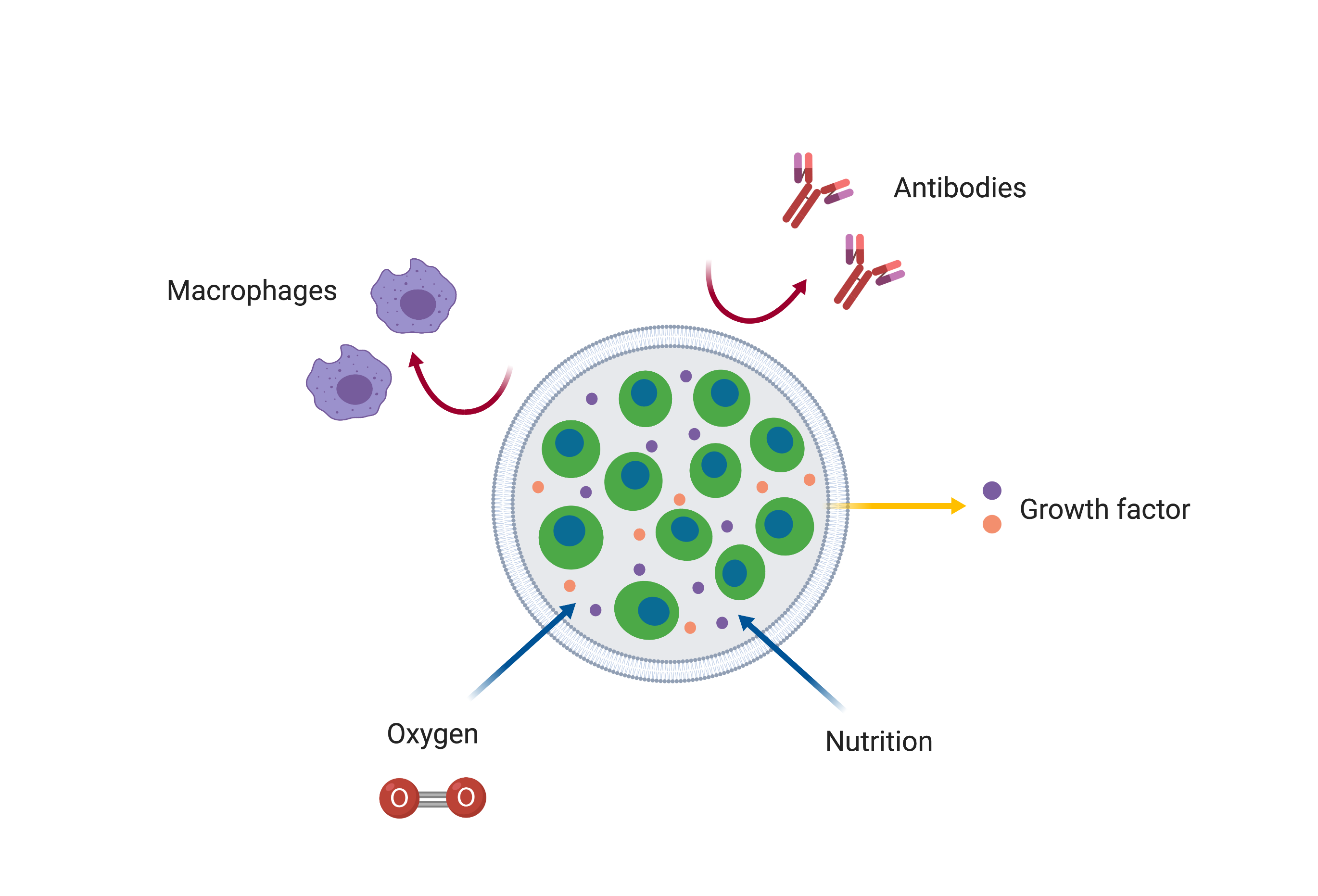
After introducing the gene circuit into the cells, the encapsulation method was chosen as the method for inserting the cells onto the skin surface.
Encapsulation method….
1. is safe because it can selectively transmit material compared to other methods (stem cells, nano-needle)
2. can limit the area where cells can grow, making it easy to make a safe and desired shape of tattoo.
