Results
Overview
Nested intein system
- Designed/synthesized/purified two sets of nested intein constructs
- Demonstrated proof-of-concept splicing of nested intein constructs in-vitro
- Characterized the (in)solubility of split-intein fusion proteins
- Designed/synthesized/purified split-linker constructs
- Demonstrated the viability of intein-based linker construction in-vitro
- Demonstrated the orthogonality of split-inteins in small linker fragments
Nested Intein Splicing Assays

As a proof of concept test for our nested intein system, we did a splicing assay of BBa_K3308083 (pVS00047) and BBa_K3308084 (pVS00048). These plasmids contain the NrdJ-1 C-terminal split between the fourth and fifth amino acid by TvoVMA. Splicing of tvoVMA inteins together will reconstitute the NrdJ-1 C terminal intein. The sequences flanking the TvoVMA intein were the unmutated NrdJ-1 C terminal residues. As demonstrated by Figure 1, at 24 hours, intein splicing is observed by the presence of the splice product.
Split-Linker Splicing Assays

As a proof of concept test for our Split-linker system, we did a splicing assay of BBa_K3308027 (pVS00044)and BBa_K3308034 (pVS00064). These plasmids contain the GP41-8 which forms the connection between the SceVMA intein and the linker. The sequences were the native flanking sequences for GP41-8. This allowed for rapid splicing to occur. A faint band is visible in the 5 second As demonstrated by Figure 1, at 24 hours, intein splicing is observed by the presence of the splice product.
Expression Tests
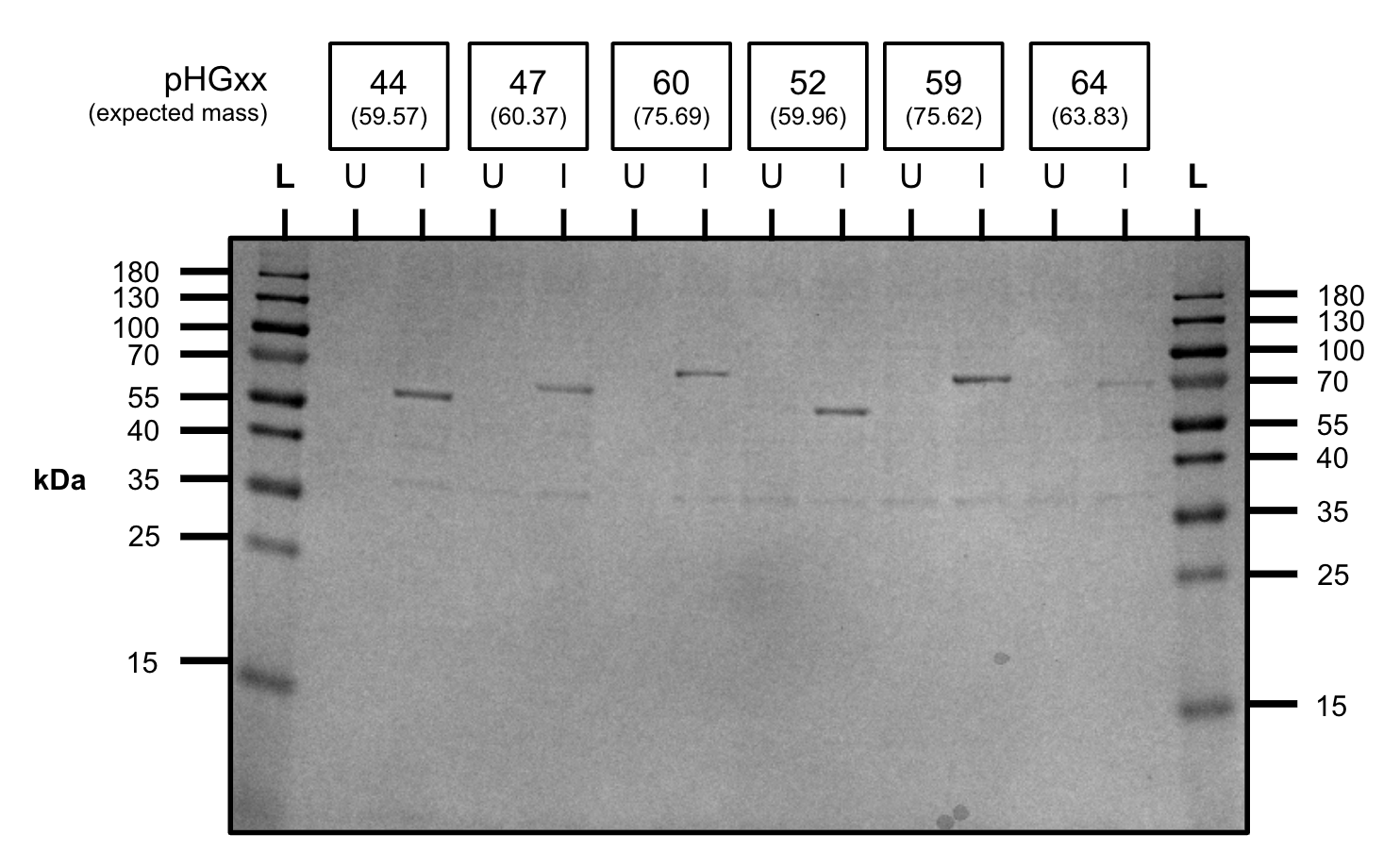
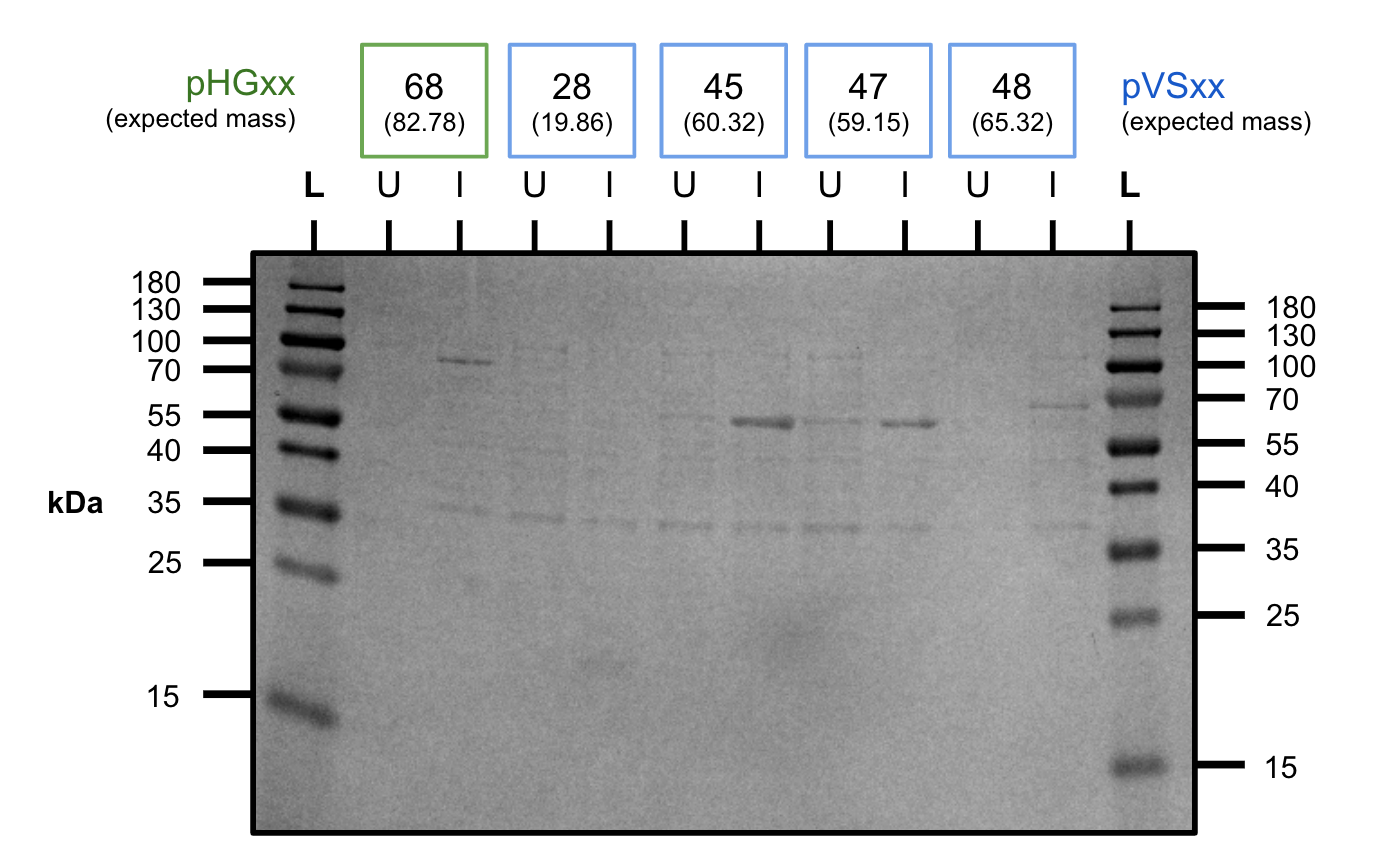
After cloning our constructs into BL21, we tested our constructs to see if we were able to see the constructs at the correct size on the SDS-PAGE. As shown above, all of our constructs showed positive expression of all of our constructs.
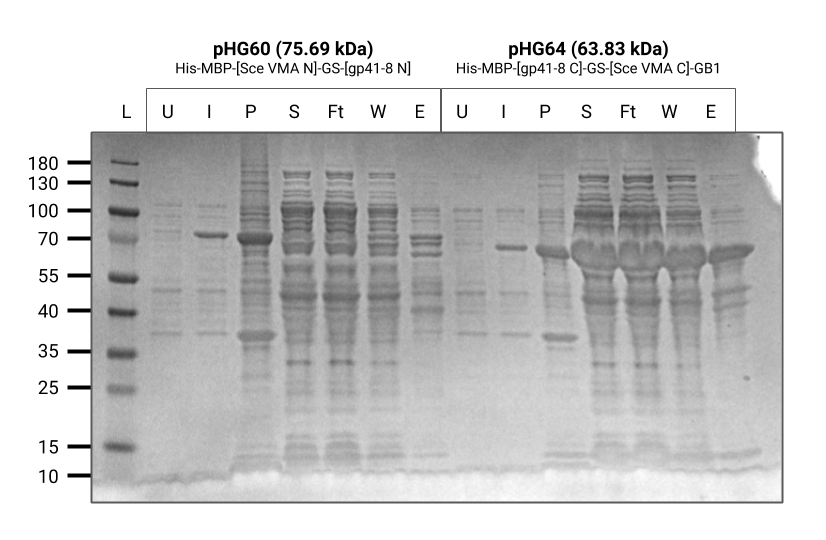
As seen in this gel, the purification of BBa_K3308034 (pVS00064) was relatively successful. There was a thick band that showed up in the elution lane of the gel. However, there seemed to be some nonspecific binding that showed up in the elution, resulting in some impurities in the gel. Our next step for this gel is the diafiltration in order to improve the purity and concentration of the protein.
