
Inspiration
Our Project Decision Roadmap:
How We Chose LACE
Before we even touched a lab bench, the first few weeks of the summer featured the six of us huddled around whiteboards and scrap paper with at least 20 tabs open at any given time as we all scoured every journal we could get our hands on for interesting project ideas. This is a walk-through of that process and how team Zorya + LACE came to be.
Why Mammalian?
Compared to bacterial systems, mammalian cell lines more accurately model human complexity and have proven to be a valuable tool in synthetic biology- increasing in prominence within biopharmaceutical manufacturing from 33% in 1989 to nearly 80% as of July 2018 [1]. Inspired by the high demand within the contemporary mammalian synthetic biology field, we decided early on to pursue a project focused on mammalian cells.
Through our research on past mammalian-based iGEM projects we were surprised to find that fewer than 5% of old iGEM teams elected to work with mammalian cells, highlighting a clear discrepancy between the demands of industry and the knowledge-base being emphasized within the iGEM community. (See supplemental materials for year-by-year breakdown)
A review of the mammalian parts within the Registry of Standard Biological Parts revealed that there were only 18 mammalian promoters available (a miniscule 4.6%) compared to over 300 bacterial promoters; this trend continues in multiple other biobrick categories as well[2]. We hypothesize that the lack of representation of mammalian synthetic biology in iGEM is due to multiple interconnected factors including the lack of standard and easily accessible protocols, time restrictions, financial limitations, and the shortage of well-characterized parts. In combination, we believe these factors both actively and subconsciously discourage iGEM teams and their advisers from pursuing mammalian projects.
In order to increase representation in this field we will need to increase overall access to these resources, beginning with the in-depth characterization of a system within multiple mammalian cell lines as a model for future projects.

To test if our hypothesized roadblocks were correct, we conducted a survey of this year's iGEM teams and their advisors. Analysis of data can be found on the Increasing Accessibility Page
Optogenetic Gene Regulation
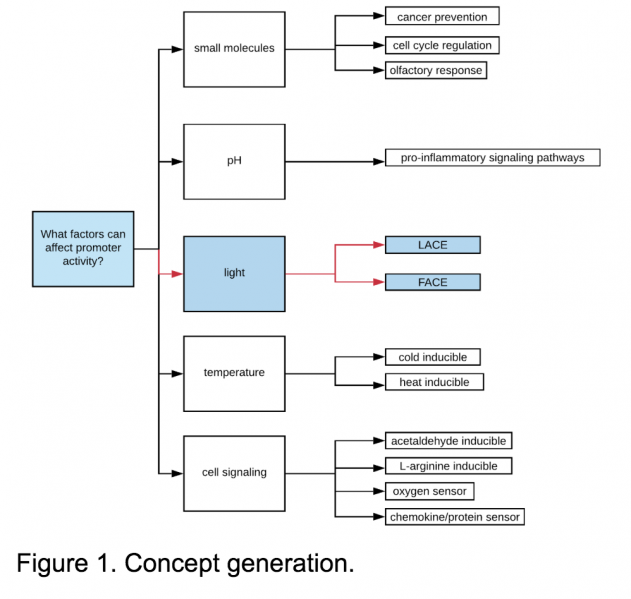
The high demand for mammalian-based research specifically targeting gene regulation within the fields of biopharmaceutical, biomedical, and biomanufacturing research directed us towards systems within this category. To narrow down what types of systems we might be interested in, we brainstormed five categories of inducible systems: small molecule inducible, pH inducible, light inducible, temperature inducible, and promoters involved in cell-signaling pathways. See Figure 1.
Light inducible systems provide spatial and temporal control over transcription[17], low toxicity to the cell [4], a large dynamic range of gene expression, reversibility [5], and decreased dependence on transcription and degradation of small molecules[5]; all of which are helpful when working with tissues which contain the additional dimension of depth.
Of the previously explored optogenetic systems, blue wavelengths of light provided the most diverse range, in addition to requiring the fewest additional co-factors, making it a logical choice.
 Figure 2. Summary of the Established Systems. [9]
Figure 2. Summary of the Established Systems. [9]
Why LACE?
After extensive literature review, we selected the LACE (Light-Activated CRISPR-dCas9 effector) system to characterize due to resource availability and team interest. This system combines characteristics of optogenetics, gene regulation, and CRISPR-based targeting within a mammalian chassis - an ideal model for further characterization, optimization, and adaptation for further application.
This system includes a pair of dimerizing proteins (CRY2 and CIBN) extracted from Arabidopsis thaliana which react in the presence of blue light. To the CIBN(s) we have attached a dCas9 complex, which binds our endogenous gene of interest as directed by our sgRNAs. To the CRY2 we have attached an activator (VP64 or VPR) to upregulate the gene of interest in the presence of blue light. See the Project Description below for more information.
Description
Meet LACE
Our project characterizes the activity of the Light Activated CRISPR-dCas9 Effector (LACE) system in the enhancement of endogenous genes within three mammalian cell lines (CHO-DG44, AML 12, and NIH-3T3). This system includes a pair of proteins, CRY2 and CIBN, extracted from Arabidopsis thaliana which heterodimerize in the presence of blue light.

The genetic anchor probe is regulated by CRISPR’s d-Cas9 complex which binds to our selected endogenous gene of interest as directed by sgRNAs, flanked by two CIBNs. Polstein et al. [18] showed that by attaching CIBN to both the N- and C- terminus of the dCas9 increased selected endogenous gene activation by 10 to 100 fold in comparison to a single CIBN.
The activator probe consists of CRY2FL, photosensory full length Cryptochrome 2, and the transcriptional activator VP-64 or VPR. In the presence of blue light, CRY2 and CIBN heterodimerize, recruiting the activator to the regulatory site of the target gene of interest.
To the CIBN(s) we have attached a dCas9 complex, which binds our endogenous gene of interest as directed by our sgRNAs. To the CRY2 we have attached an activator (VP64 or VPR) to upregulate the gene of interest under blue light conditions.

Mammalian Cell Transfections
Lipofectamine 3000 transfection efficiency was optimized in all three cell lines using pMAX as well as a light activated three plasmid system with LACE sgRNAs targeting a GFP plasmid. Cell density and GFP expression was calculated both using UV imaging with ImageJ data processing as well as flow cytometry. (see imaging and flow cytometry protocol)
Due to the multiplicative decrease of transfecting a two plasmid system vs a single GFP plasmid, transfection efficiency required further optimization. Seeding density, lipofectamine 3000 concentration, DNA concentration, light intensity, an additional wash step, and plasmid ratios were all varied to produce the optimal conditions for each cell line. (see transfection optimization protocol)

Bright Field transfected AML-12 Cells

pMAX GFP transfected AML-12 Cells

Construction of the Light Box

Methods of emitting and measuring light are not standardized between labs and produced significant variety between optogenetic systems found in literature. The two light boxes we constructed and used for blue light exposure of the LACE system were based on the open-access designs published by the Tabor Lab at Rice under the guidance of Huynsoo Kim and Steven Lucero.
To calibrate the light box and compensate for slight manufacturing variations of LED intensity, each well was measured for a lux value, using a recreational lux meter. These values were recorded and specific text files, used by the microcontroller of the LPA, were edited in order to make each well emit similar values of lux. See Hardware for protocols and further information.
Dose Response Curves
When characterizing the effects of a system it is vital to understand its response under different intensities and frequencies for use in future applications, similar to the dose response curves from chemical inducers. By creating a detailed dosage response curve for the LACE system, we will be documenting general trends and enabling future researchers to better select the optimal conditions for their experiment. Too high of an intensity or frequency might lead to increased heat that would be detrimental to cell health, whereas settings that are too low might not be enough to induce dimerization of the two light sensitive proteins.
We have designed two tests as part of our “Time Trials”: a 5 hour system initiation test and a 24 hour system longevity test to track internal mRNA levels.



















 [17]
[17]








