
Our Project
Our Project
Background
In 1906, squalene, a triterpenoid, was found in shark liver oil. Since then, squalene has long been given considerable attention because of its medical values. It has been found in human beings, animals, plants and microorganism.
Squalene is a universal precursor for many bioactive compounds, such as sterols, cholesterols and terpenoids. Being alible and preventive to diseases, squalene enjoys unique physical and chemical properties, making it beneficial to humans’ health and lives. For example, taking in squalene as nourishment can reduce the blood cholesterol level, prevent coronary artery disease and cancer, enhance the antitumor effect of chemotherapy drugs and improve the immune system.
Furthermore, squalene is widely used in cosmetic industry as a moisturizer and emollient due to its antioxidant properties, and it can also protect the skin from shortwave radiation; in pharmaceutical and medical fields, squalene-based emulsion can be effectively used in vaccines and drug delivery. It improves the absorption of active ingredients by cells due to the reduction of active ingredients’ release rate. The biocompatibility of squalene allows it to be used to treat bacterial and fungal infections of the skin. Squalene has also been found to be a source of biofuels.
It was found that squalene content in livers of deep-sea sharks is the highest on earth, which reaches 80%. Shark liver oil is the best natural source of squalene. However, thanks to the impact on marine environment posed by continuous organic pollutants, intensive shark hunting, and the public attention on marine animal’s protection, the exportation and importation of shark liver oil has decreased, so the squalene’s source has been limited, in the past twenty years. Though squalene can be obtained in plant products like the olive oil, amaranth seed oil, and palm fatty acid, yet this method features a longer growing time, lower efficiency, higher costs, and an unsteady producing process, which may not satisfy the industrial and pharmaceutical needs. Moreover, microorganisms are also a valuable source. Some of the microorganisms, nevertheless, have a low squalene yield and a rather difficult process to have them genetic engineered. Also, they have never been used before. That’s why these microorganisms are not appropriate to large-scaled industrial productions.

Figure1. Potential applications and natural sources of squalene [10]. (A) Potential applications of squalene for treatment of cancer, as detoxifier, use in cosmetics, drug and vaccine adjuvants. (B) Potential natural sources of squalene. Figure depicts the possible squalene sources ranging from unicellular microbes such as yeast and other bacterial cells to multicellular fungi, plants, and deep sea sharks. All these sources can produce squalene through mevalonate pathway. DCW, Dry cell weight.
E. coli and saccharomyces cerevisiae have the advantages of rapid growth, clear background, simple and mature genetic manipulation system, safety and easy large-scale cultivation, thus by using rational design, efficient component integration and assembly technology of synthetic biology the synthesis pathway can be rapidly rebuilt in microorganism hosts. Saccharomyces cerevisiae, having all the genes needed to synthesize triterpenoids (Ergosterol), is an excellent genetic engineering host to synthesize squalene. However, other newly introduced triterpenoid synthesis routes will compete and cross-react with ergosterol synthesis route. Since endogenous ergosterol pathway is necessary for the survival of s. cerevisiae and thus cannot be completely deleted, the residual ergosterol synthetic pathway will affect the allogeneic expression of genes in the squalene synthesis pathway, resulting in the decrease of the actual production and productivity of squalene, so hosts lacking triterpene synthetic pathway are more attractive. Because E. coli does not synthesize endogenous triterpenoids, biosynthetic squalene can accumulate stably in E. coli without being consumed or transformed into other unwanted compounds. Therefore, E. coli is an ideal genetic engineering host for the biosynthesis of squalene.
There have been some progress in synthesizing squalene with E. coli, in recent years, in which the yield result has reached 230mg/L. This was accomplished only by MVA pathway according to the report, so we considered that there would still be much room for improvement, which is also one of the reasons why we have decided to conduct this project.
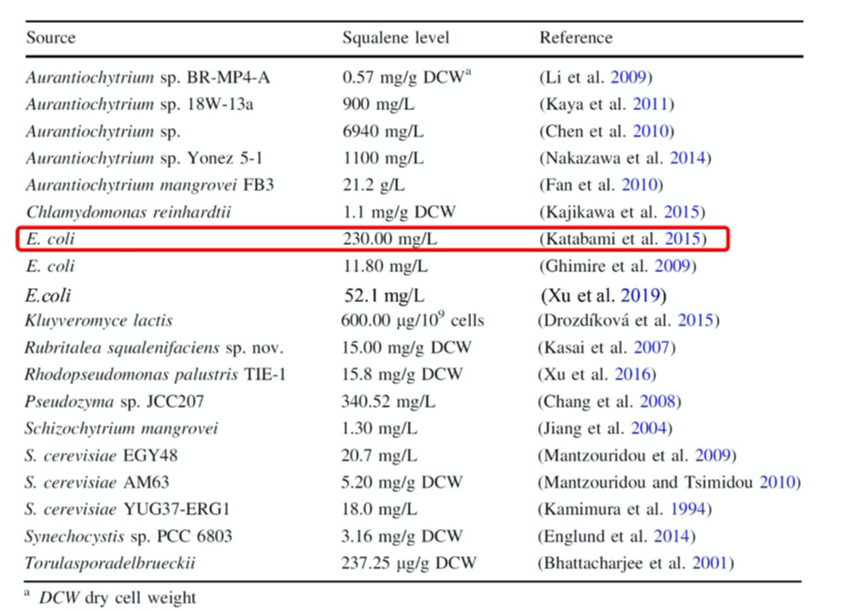
Figure.2 Production of squalene in various microorganisms
The Synthesis Pathway of Squalene
In nature, two independent pathways are responsible for the biosynthesis of common isoprenoid precursors isopentenyl/dimethylallyl diphosphate (IPP/DMAPP), 2-C-methyld-erythritol-4-phosphate pathway (MEP pathway, mainly acts on eubacteria and plant plastids) and mevalonate pathway (MVA pathway, mainly acts on plant cytosols and animals).
The MVA pathway begins with the condensation of acetyl-CoA molecules to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), and then reduces to produce mevalonate. Subsequently, mevalonate is converted to IPP through three reactions that involve ATP phosphorylation and decarboxylation. An IPP isomerase catalyzes the interconversion between IPP and DMAPP. The MEP pathway begin with the condensation of glyceraldehyde 3-phosphate (GAP) and pyruvate and MEP are produced by reductive isomerization reaction. Subsequent connection with CTP, phosphorylation, cyclization, and two reductive dehydration steps produce DMAPP and IPP.
Squalene is biosynthesized by the head-to-head condensation of two farnesyl diphosphate (FPP) molecules catalyzed by the single enzyme squalene synthase (SQS). Farnesyl diphosphates are available in E. coli. Therefore, squalene production in E. coli is achieved simply by expressing SQS.

Figure.3 Endogenous MEP and synthetic pathways for squalene. The MEP pathway is the endogenous isoprenoid pathway found in E. coli. Using farnesyl diphosphate as the starting material, squalene is produced by exogenously expressed squalene synthases (SQSs).
In order to improve terpenoid production, many metabolic engineering efforts have been targeting at the MVA and MEP pathways to increase IPP and DMAPP supply in host microorganisms. So far, the introduction of MVA has been shown to be much better in practice than the endogenous MEP pathway in E. coli in that this way the MEP-associated native regulatory mechanism, which has not yet been completely characterized and understood due to its complex nature, can be avoided. In spite of the fact that the MVA pathway has been more successful, it has been proved by previous studies through modeling, that MEP pathway theoretically has a higher yield of isoprenoids in E. coli. Because of this, various studies have been carried out in the last decade, aiming at improving the flux of MEP pathway.
So far, 1-deoxy-d-xylulose-5-phosphate synthase gene (dxs) and isopentenyl-isomerase gene (idi) are known to be the most important game changers in enhancing IPP/DMAPP flux. They mainly contribute to catalyzing reactions that were commonly viewed as the major rate-limiting steps.
MEP pathway genes were regulated to investigate whether the pathway contains new rate-limiting steps and toxic intermediates. It was reported that the activation of IspG leads to cell growth and the significance drop of beta carotene production. It was found that the overexpression of ispG led to the accumulation of intermediate HMBPP, which seriously interfered with the synthesis mechanism of E. coli nucleotides and proteins. HMBPP accumulation could be solved by activation of downstream enzyme IspH and eliminate the negative effect of ispG overexpression. In addition, the intermediate MECPP cumulative strain, can balance the activation of IspG and IspH, promoting carbon flux from MECPP and leading to beta carotene and lycopene titer increase by 73% and 77% respectively. The results showed that the balanced activation of IspG and IspH could eliminate the accumulation of HMBPP and MECPP and increase the yield of isoprene.
The difference between host strains may also affect the synthesis of target products. Lycopene production of 3 K-series E. coli strains was significantly different in wild type/engineered (overexpression of dxs) hosts (XL1-blue > DH5 > JM101).
Our method is to employ various tactics of optimization comprehensively. In this work, we designed MEP and MVA pathways in E. coli to increase squalene production.

