Cecileprince (Talk | contribs) |
Cecileprince (Talk | contribs) |
||
| Line 203: | Line 203: | ||
<h1 class="mt-5">References</h1> | <h1 class="mt-5">References</h1> | ||
| − | <br>[1] Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother (2006) 60, 502-507. | + | <br><p>[1] Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother (2006) 60, 502-507.</p> |
| − | <br>[2] Andrade JC, Rocha-Santos TAP, Duarte AC, Gomes AM, Freitas AC. Chapter 4 - Biotechnological Production of Conjugated Fatty Acids With Biological Properties. In Handbook of Food Bioengineering, Food Bioconversion. Grumezescu AM, Holban AM, Eds. Academic Press (2017) pp. 127-178. | + | <br><p>[2] Andrade JC, Rocha-Santos TAP, Duarte AC, Gomes AM, Freitas AC. Chapter 4 - Biotechnological Production of Conjugated Fatty Acids With Biological Properties. In Handbook of Food Bioengineering, Food Bioconversion. Grumezescu AM, Holban AM, Eds. Academic Press (2017) pp. 127-178.</p> |
| − | <br>[3] Cao, Y. et al. Re-characterization of three conjugated linolenic acid isomers by GC–MS and NMR. Chemistry and Physics of Lipids 145, 128–133 (2007). | + | <br><p>[3] Cao, Y. et al. Re-characterization of three conjugated linolenic acid isomers by GC–MS and NMR. Chemistry and Physics of Lipids 145, 128–133 (2007).</p> |
| − | <br>[4] Sassano, G. et al. Analysis of pomegranate seed oil for the presence of jacaric acid. J. Sci. Food Agric. 89, 1046–1052 (2009). | + | <br><p>[4] Sassano, G. et al. Analysis of pomegranate seed oil for the presence of jacaric acid. J. Sci. Food Agric. 89, 1046–1052 (2009).</p> |
| − | <br>[5] Fritsche, K., Hornung, E., Peitzsch, N., Renz, A. & Feussner, I. Isolation and characterization of a calendic acid producing (8,11)-linoleoyl desaturase 1. FEBS Letters 462, 249–253. | + | <br><p>[5] Fritsche, K., Hornung, E., Peitzsch, N., Renz, A. & Feussner, I. Isolation and characterization of a calendic acid producing (8,11)-linoleoyl desaturase 1. FEBS Letters 462, 249–253.</p> |
| − | <br>[6] Cao, Y., Gao, H.-L., Chen, J.-N., Chen, Z.-Y. & Yang, L. Identification and Characterization of Conjugated Linolenic Acid Isomers by Ag + -HPLC and NMR. J. Agric. Food Chem. 54, 9004–9009 (2006). | + | <br><p>[6] Cao, Y., Gao, H.-L., Chen, J.-N., Chen, Z.-Y. & Yang, L. Identification and Characterization of Conjugated Linolenic Acid Isomers by Ag + -HPLC and NMR. J. Agric. Food Chem. 54, 9004–9009 (2006).</p> |
| − | <br>[7] Shabbir MA, Khan MR, Saeed M, Pasha I, Khalil AA, Siraj N. Punicic acid: A striking health substance to combat metabolic syndromes in humans. Lipids Health Dis. (2017) 16, 99. | + | <br><p>[7] Shabbir MA, Khan MR, Saeed M, Pasha I, Khalil AA, Siraj N. Punicic acid: A striking health substance to combat metabolic syndromes in humans. Lipids Health Dis. (2017) 16, 99. </p> |
| − | <br>[8] Holic R, Xu Y, Caldo KMP, Singer SD, Field CJ, Weselake RJ, Chen G. Bioactivity and biotechnological production of punicic acid. Appl Microbiol Biotechnol (2018) 102, 3537-3549. | + | <br><p>[8] Holic R, Xu Y, Caldo KMP, Singer SD, Field CJ, Weselake RJ, Chen G. Bioactivity and biotechnological production of punicic acid. Appl Microbiol Biotechnol (2018) 102, 3537-3549.</p> |
| − | <br>[9] Zhang B, Chen H, Li M, Gu Z, Song Y, Ratledge C, Chen YQ, Zhang H, Chen W. Genetic engineering of <i>Yarrowia lipolytica</i> for enhanced production of trans-10, cis-12 conjugated linoleic acid. Microb Cell Fact (2013) 12, 70. | + | <br><p>[9] Zhang B, Chen H, Li M, Gu Z, Song Y, Ratledge C, Chen YQ, Zhang H, Chen W. Genetic engineering of <i>Yarrowia lipolytica</i> for enhanced production of trans-10, cis-12 conjugated linoleic acid. Microb Cell Fact (2013) 12, 70.</p> |
| − | <br>[10] Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicaud JM. <i>Yarrowia lipolytica</i> as a model for bio-oil production. Prog Lipid Res (2009) 48, 375-387. | + | <br><p>[10] Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicaud JM. <i>Yarrowia lipolytica</i> as a model for bio-oil production. Prog Lipid Res (2009) 48, 375-387.</p> |
| − | <br>[11] Ledesma-Amaro R, Nicaud JM. <i>Yarrowia lipolytica</i> as a biotechnological chassis to produce usual and unusual fatty acids. Prog Lipid Res (2016) 61, 40-50. | + | <br><p>[11] Ledesma-Amaro R, Nicaud JM. <i>Yarrowia lipolytica</i> as a biotechnological chassis to produce usual and unusual fatty acids. Prog Lipid Res (2016) 61, 40-50.</p> |
| − | <br>[12] Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicaud JM. <i>Yarrowia lipolytica</i> as a model for bio-oil production. Prog Lipid Res (2009) 48, 375-387. | + | <br><p>[12] Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicaud JM. <i>Yarrowia lipolytica</i> as a model for bio-oil production. Prog Lipid Res (2009) 48, 375-387.</p> |
| − | <br>[13] Hornung E, Pernstich C, Feussner I. Formation of conjugated Delta11Delta13-double bonds by Delta12-linoleic acid (1,4)-acyl-lipid-desaturase in pomegranate seeds. Eur J Biochem (2002) 269, 4852-4859. | + | <br><p>[13] Hornung E, Pernstich C, Feussner I. Formation of conjugated Delta11Delta13-double bonds by Delta12-linoleic acid (1,4)-acyl-lipid-desaturase in pomegranate seeds. Eur J Biochem (2002) 269, 4852-4859.</p> |
| − | + | ||
<br> | <br> | ||
| − | <br>< | + | <br> |
| + | |||
| + | </div> | ||
</div> | </div> | ||
</body> | </body> | ||
Revision as of 01:10, 22 October 2019

Plants have a power!
Humans have always been eating plants, but not many people know the real powers plants have! A big part of their wealth lies in the vitamins and essential oils they produce. They also contain many active molecules that we, as humans, have been using for medical purposes. In that sense, nature is a huge source of knowledge. In order to properly use nature we need to carefully study it to finely understand its true essence. Technologies based on lack of knowledge lead us to over-exploitation, deforestation, forest fires, and more generally to global warming that is changing ecosystems. We are dangerously close to losing some of the knowledge nature can give us. That's why we have developed a heightened sense of interest in it.
We have noticed that plants contain many interesting molecules, and that some of them are essential to our survival because our bodies cannot produce them by ourselves. Among them are unsaturated fatty acids.
Fatty acids are present on the cell membranes of our body and play a major role in tissue signaling and participate in the energy metabolism in the formation of ATP. Our diet is therefore our only source to get these fatty acids; two of them are essential precursors: linoleic acid as Omega-3 and linolenic acid as Omega-6 [1].
These fatty acids are essential to our survival, and keeping a balance between the two families of fatty acids seems to be a secret (not so secret) of longevity. As a matter of fact, there are disparities between regions of the world about the Omega-6/ Omega-3 ratio which can lead to inflammatory phenomena or cancer induction, among other complications [1].
In addition to these two essential fatty acids, other unsaturated fatty acid molecules are studied because of their medicinal properties.
Then as we can see, fatty acids have interesting properties and are even essential !
The main difficulties encountered in the production of these fatty acids is their high cost of production, and the negative impact it has on the plants these molecules are extracted from. That is why we wanted to focus our project on the synthesis of these rare unsaturated fatty acids. Our goal is to synthesize unsaturated fatty acids using a modified yeast strain as chassis. In order to know if our project was feasible and viable, we questioned ourselves about the means needed to succeed in our approach and the different consequences resulting from such a project. We performed a “Human Practice” study to address the different environmental, economic, legal, political and social issues linked to the production of rare fatty acids. For this, we have deepened the analysis and met professionals who played a major role in our project.
One of their secret keys
Their double bonds! Our team is interested in unusual fatty acids and in particular Conjugated Linolenic Acids (CLnA) [2].
| Name | Nomenclature | Structure | Reference |
|---|---|---|---|
| Alpha-eleostearic acid | C18:3 (9Z, 11E, 13E) |  |
|
| Beta-eleostearic acid | C18:3 (9Z, 11Z, 13Z) |  |
|
| Catalpic acid | C18:3 (9Z, 11Z, 13E) |  |
|
| Alpha-calendic acid | C18:3 (8E, 10E, 12Z) |  |
|
| Beta-calendic acid | C18:3 (8E, 10E, 12E) |  |
|
| Jacaric acid | C18:3 (8Z, 10E, 12Z) |  |
|
| Punicic acid | C18:3 (9Z, 11E, 13Z) |  |

This diversity on structure (position of these 3 double bonds and their conformation) led us to develop our project on Jacaranda mimosifolia, also called "Blue Jacaranda", which is a famous tree that grows in tropical countries and is characterized by its magnificent blue flowers. This tree is also known for its anti-microbial properties and has been used in folk medicine by people living in regions where this tree grows.
Another “flashy” plant is the pomegranate (Punica granatum). Nowadays, it is very famous among people who eat healthy food, but its benefits on health are not well known outside of these groups.
Knowing their interesting properties, we decided to use synthetic biology to produce molecules contained in these plants.
These fatty acids are characterized by their three conjugated bonds and have a large number of medical applications. The anti-tumor, anti-obesity and anti-inflammatory properties of two CLnA, jacaric acid and punicic acid have been clearly demonstrated [7].
Our fat-bulous project:
However, any large-scale production of rare fatty acids would necessarily lead to new plantation, and therefore environmental problems. The use of synthetic biology for production could help avoid a potential overexploitation of these plants. Protection of the environment is one of our objectives, one of the major issues of our time. We do not want to repeat the mistakes of the past. The exploitation of palm oil is sadly lucrative for the exploiter because of its high value. This problem applies equally well to Punica granatum and Jacaranda mimosifolia, that's why we chose those two for this project. We want to produce these rare fatty acids without having an adverse impact on the environment. Since these types of fatty acids are present in very small quantity in the plants, bio-production can be even more important to prevent large scale deforestation [8].

Our Synthetic biology project is therefore very adapted to deal with the problems of deforestation. Especially for projects that wish to exploit plants for industrials pharmaceuticals, agrofood and cosmetics. Indeed, bio-production through microorganisms requires less resources, less space for a proportionally higher yield. So our project would allow us to start developing alternatives to deforestation.
Our objective is to develop a platform for rare fatty acids bioproduction, in order to limit environment and economic problems in the long term. As a biological chassis, we will use yeast and especially oily yeast Yarrowia lipolytica because it is a species that has already proven its effectiveness for the production of fatty acids, thanks to its highly developed lipid metabolism. [9] [10] [11].
Yarrowia: an ideal chassis for fatty acid production

However, as part of our biomanufacturing project, we have chosen to use the yeast Yarrowia lipolytica. Our choice was motivated by several elements that make Yarrowia an ideal chassis for the bio-production of rare fatty acids also known as conjugated linoleic acids:
Yarrowia lipolytica is a GRAS organism (generally recognized as safe), so it is safe and easy to handle.
Yarrowia lipolytica has a metabolism capable of producing linoleic acid, which is the substrate for the synthesis of Conjugated linolenic acids (CLnA) we want to produce [11].
Yarrowia lipolytica is one of the rare oleaginous organisms capable of storing large amounts of fatty acids in its cytoplasm. Up to 40% of the cytoplasmic volume can be allocated to the storage of fatty acids [12].
Therefore punicic acid synthesis could be used as a trial to prove the effectiveness of our design, before attacking the more troublesome case of jacaric acid. Our first objective is the elaboration of our production platform for synthesis of punicic acid. Our second objective is to prepare in parallel the baseline for the production of jacaric acid. Consequently, we wanted to find the enzyme that catalyzes the reaction of synthesis of jacaric acid. To do so, we decided to sequence the entire exome of the only tree known to date to produce this fatty acid, Jacaranda mimosifolia. Through bioinformatic analysis of the subsequent data, we will successfully determined the sequence of the missing enzyme.
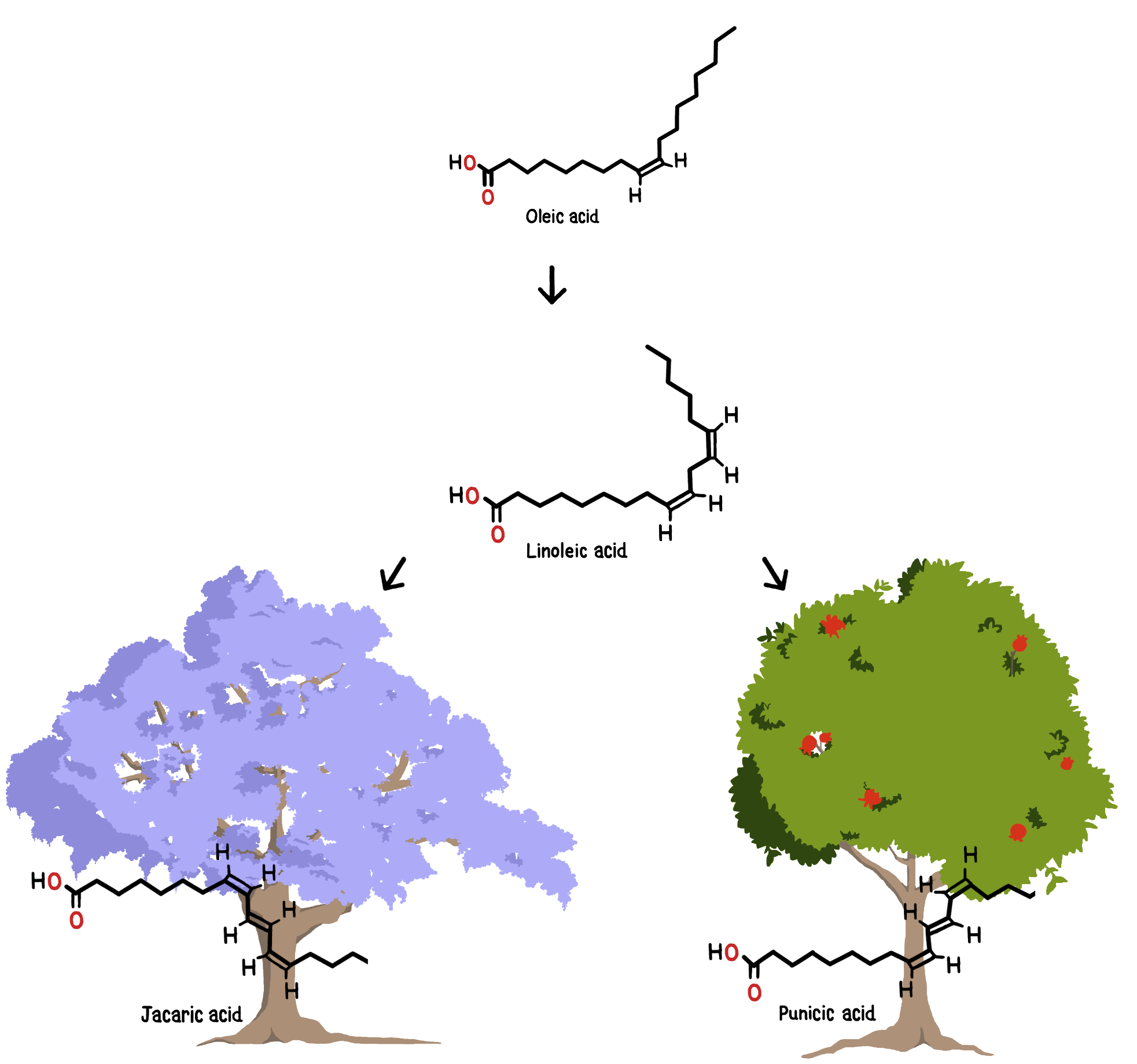

However, Yarrowia lipolytica does not naturally produce the rare fatty acids we are interested in. So, we need to find the enzymes that allow the synthesis of these fatty acids. As a matter of fact, we were able to identify relatively easily the protein sequences of the enzyme catalyzing the formation of punicic acid from linoleic acid [13]. However, the search for enzymes that allow the synthesis of jacaric acid was harder. After a long search we have not been able to obtain this sequence, so we conclude that it is not known yet.
References
[1] Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother (2006) 60, 502-507.
[2] Andrade JC, Rocha-Santos TAP, Duarte AC, Gomes AM, Freitas AC. Chapter 4 - Biotechnological Production of Conjugated Fatty Acids With Biological Properties. In Handbook of Food Bioengineering, Food Bioconversion. Grumezescu AM, Holban AM, Eds. Academic Press (2017) pp. 127-178.
[3] Cao, Y. et al. Re-characterization of three conjugated linolenic acid isomers by GC–MS and NMR. Chemistry and Physics of Lipids 145, 128–133 (2007).
[4] Sassano, G. et al. Analysis of pomegranate seed oil for the presence of jacaric acid. J. Sci. Food Agric. 89, 1046–1052 (2009).
[5] Fritsche, K., Hornung, E., Peitzsch, N., Renz, A. & Feussner, I. Isolation and characterization of a calendic acid producing (8,11)-linoleoyl desaturase 1. FEBS Letters 462, 249–253.
[6] Cao, Y., Gao, H.-L., Chen, J.-N., Chen, Z.-Y. & Yang, L. Identification and Characterization of Conjugated Linolenic Acid Isomers by Ag + -HPLC and NMR. J. Agric. Food Chem. 54, 9004–9009 (2006).
[7] Shabbir MA, Khan MR, Saeed M, Pasha I, Khalil AA, Siraj N. Punicic acid: A striking health substance to combat metabolic syndromes in humans. Lipids Health Dis. (2017) 16, 99.
[8] Holic R, Xu Y, Caldo KMP, Singer SD, Field CJ, Weselake RJ, Chen G. Bioactivity and biotechnological production of punicic acid. Appl Microbiol Biotechnol (2018) 102, 3537-3549.
[9] Zhang B, Chen H, Li M, Gu Z, Song Y, Ratledge C, Chen YQ, Zhang H, Chen W. Genetic engineering of Yarrowia lipolytica for enhanced production of trans-10, cis-12 conjugated linoleic acid. Microb Cell Fact (2013) 12, 70.
[10] Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicaud JM. Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res (2009) 48, 375-387.
[11] Ledesma-Amaro R, Nicaud JM. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog Lipid Res (2016) 61, 40-50.
[12] Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicaud JM. Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res (2009) 48, 375-387.
[13] Hornung E, Pernstich C, Feussner I. Formation of conjugated Delta11Delta13-double bonds by Delta12-linoleic acid (1,4)-acyl-lipid-desaturase in pomegranate seeds. Eur J Biochem (2002) 269, 4852-4859.




















