
Choose genes and expression vector

General description
As we mentioned before, to focus on cellulose and get high quality banana fiber, the most important work is removing lignin and pectin. But what kind of genes should we choose? After consulting papers, we choose pectin lyase A (pelA), small laccase (slac) and versatile peroxide (VP) to achieve our goal.
pelA: Pectin lyase A was found in Aspergillus nigra EIM-6. It can break ester bonds in pectin. We use it to deal with the pectin which cover the surface of the fiber. And it will generate the galacturonic acids.
The lyase acts on the fifth position β carbon atom of galacturonic acid by β racemization, so that the H on the β carbon atom is transferred to the oxygen atom of the glycosidic bond, and the glycosidic bond is broken, forming a double bond between C4 and C5 of galacturonic acid. It produces galacturonic acid oligosaccharide.
VP: Versatile peroxidase was found in Pleurotus ostreatus. Versatile peroxidase is a kind of peroxidase comprising activities of manganese and lignin peroxidases. It has interaction sites of those two. They have different mechanisms to decompose the lignin, but they oxidize it first. The oxidized lignin generates unstable free radicals and then undergo a series of non-enzymatic spontaneous cracking reactions, leading to oxidation and fracture of lignin polymers.

SLAC: Small laccase catalyzes oxidation of most model compounds of lignin with free phenols. The catalytic reaction contains four consecutive one-electron oxidations, oxidizing the phenol ring to generate the phenoxy group, an intermediate from the radical. Phenoxy group is a cationic group with oxygen as the center. It can usually undergo non-enzyme-catalyzed cleavage reaction, which eventually leads to the bond breaking between aryl group and α carbon atom.
We also need an appropriate expression vector. After discussion, we chose Pichia pastoris to be our chassis because of its clear and simple mechanism and strong ability to express enzyme. Pichia pastoris has been used in synthetic biology field for a long time because of its expression advantages. This time, we chose pichia pastoris because exogenous protein can be expressed in pichia pastoris with high quality and sizable quantity.

pelA: Pectin lyase A was found in Aspergillus nigra EIM-6. It can break ester bonds in pectin. We use it to deal with the pectin which cover the surface of the fiber. And it will generate the galacturonic acids.
The lyase acts on the fifth position β carbon atom of galacturonic acid by β racemization, so that the H on the β carbon atom is transferred to the oxygen atom of the glycosidic bond, and the glycosidic bond is broken, forming a double bond between C4 and C5 of galacturonic acid. It produces galacturonic acid oligosaccharide.
VP: Versatile peroxidase was found in Pleurotus ostreatus. Versatile peroxidase is a kind of peroxidase comprising activities of manganese and lignin peroxidases. It has interaction sites of those two. They have different mechanisms to decompose the lignin, but they oxidize it first. The oxidized lignin generates unstable free radicals and then undergo a series of non-enzymatic spontaneous cracking reactions, leading to oxidation and fracture of lignin polymers.

SLAC: Small laccase catalyzes oxidation of most model compounds of lignin with free phenols. The catalytic reaction contains four consecutive one-electron oxidations, oxidizing the phenol ring to generate the phenoxy group, an intermediate from the radical. Phenoxy group is a cationic group with oxygen as the center. It can usually undergo non-enzyme-catalyzed cleavage reaction, which eventually leads to the bond breaking between aryl group and α carbon atom.
We also need an appropriate expression vector. After discussion, we chose Pichia pastoris to be our chassis because of its clear and simple mechanism and strong ability to express enzyme. Pichia pastoris has been used in synthetic biology field for a long time because of its expression advantages. This time, we chose pichia pastoris because exogenous protein can be expressed in pichia pastoris with high quality and sizable quantity.

Parts Design
In our design, to get these enzymes secreted out of cells to decompose pectin and lignin, we need a signal peptide to combine with each enzyme to achieve the transmembrane transportation of enzyme. That’s why we insert a piece of signal peptide sequence.
And to control whether parts work or not, AOX1 promoter, a methanol inducible promoter, was used. We also add a His-Tag in its tail. His-Tag can add several special amino acids that chelate metal ions and make the proteins easier to be attached to chromatography media. In other words, it will be easier to purify and collect the proteins.
In order to develop an efficient Bananas’ castoff-process system, we want Pichia pastoris to secrete more enzymes, so we find six different signal peptides from literatures, link each signal peptide sequence to each enzyme gene and get eighteen combinations. In this step, we transfer every combination into expression vector respectively, and add methanol to induce the expression, and measure the enzyme activity. In this way, we can know which combination can produce the enzymes with highest activity.

And to control whether parts work or not, AOX1 promoter, a methanol inducible promoter, was used. We also add a His-Tag in its tail. His-Tag can add several special amino acids that chelate metal ions and make the proteins easier to be attached to chromatography media. In other words, it will be easier to purify and collect the proteins.
In order to develop an efficient Bananas’ castoff-process system, we want Pichia pastoris to secrete more enzymes, so we find six different signal peptides from literatures, link each signal peptide sequence to each enzyme gene and get eighteen combinations. In this step, we transfer every combination into expression vector respectively, and add methanol to induce the expression, and measure the enzyme activity. In this way, we can know which combination can produce the enzymes with highest activity.

System development
We have designed our parts and determined enzyme activity, but just linking them together is not enough. The problem lies that the product of pectin degradation is galacturonic acid, which will decline pH. In that case, what we need is a pH feedback regulation system, which will enable us to regulate pH. We will explain how we design this system and how it works as follow.
We choose pGAP promoter (This promotor does not need to induce expression) instead of AOX1 as the promoter of pelA gene considering of the usage in industry, because adding extra material into fermenter tank regularly means higher financial and labor cost. PgpdA promoter (a promoter works well when pH lower than 5) replaces AOX1 as the promoter of VP gene and SLAC gene. There is a new alkali gene with pgas promoter, which will express when pH is near 2.
Our design is about one device including four parts.

For starter, we chose the promoter pGAP to initialize the whole system. The promotor can initialize the expression of the first enzyme, pela, pectin lyase. It can hydrolyze the pectin into galacturonic acid, which is acidic. The pH value will get lower and lower.
When it reaches 5, the promoter PgpdA will be activated, and initialize the expression of versatile peroxidase and small laccase. Both of them are oxidases that can oxidize the lignin, and the combination will enhance the efficiency. Since there are many ether bonds in lignin, its oxidation will generate a lot of carboxyl groups and continue getting PH value decrease.
We cannot let pH get too low for Pichia pastoris to survive, so we’re going to need a regulator to increase the pH value again. That’s why we add the fourth part. Once the pH value reaches 2, then this promoter Pgas will be activated and highly expressed an alkali protein. In a word, if the system works, it will repeat this circulation until all the lignin and pectin are oxidized or decomposed.
To show whether the promotor works,we add green fluorescent protein instead of pelA, VP and SLAC after the promoters as a reporter.

We choose pGAP promoter (This promotor does not need to induce expression) instead of AOX1 as the promoter of pelA gene considering of the usage in industry, because adding extra material into fermenter tank regularly means higher financial and labor cost. PgpdA promoter (a promoter works well when pH lower than 5) replaces AOX1 as the promoter of VP gene and SLAC gene. There is a new alkali gene with pgas promoter, which will express when pH is near 2.
Our design is about one device including four parts.

For starter, we chose the promoter pGAP to initialize the whole system. The promotor can initialize the expression of the first enzyme, pela, pectin lyase. It can hydrolyze the pectin into galacturonic acid, which is acidic. The pH value will get lower and lower.
When it reaches 5, the promoter PgpdA will be activated, and initialize the expression of versatile peroxidase and small laccase. Both of them are oxidases that can oxidize the lignin, and the combination will enhance the efficiency. Since there are many ether bonds in lignin, its oxidation will generate a lot of carboxyl groups and continue getting PH value decrease.
We cannot let pH get too low for Pichia pastoris to survive, so we’re going to need a regulator to increase the pH value again. That’s why we add the fourth part. Once the pH value reaches 2, then this promoter Pgas will be activated and highly expressed an alkali protein. In a word, if the system works, it will repeat this circulation until all the lignin and pectin are oxidized or decomposed.
To show whether the promotor works,we add green fluorescent protein instead of pelA, VP and SLAC after the promoters as a reporter.

MODEL
To find the most suitable expression level instead of the highest, we build a model to stimulate the whole process and tend to find the match that saves as more substrates as possible. If you want to learn more, please move to the MODEL page.
Fermentation
Fermentation is an inevitable part in real industry. So we simulated the fermentation in the laboratory, by reducing its scale. First of all, we use physical way to roll the fiber, making it more loose. Then we boiled it at 120 Centigrade(240 Fahrenheit) for 20 minutes, keeping it at a high temperature and moisture circumstances. To let the fiber dry thoroughly, we put it into the oven and dry it for 60 Centigrade(140 Fahrenheit). We set different numbers of fiber, concentration of Pichia pastoris, adding 2 milliliter each day for 22 days. After fermentation, we compared the weight of before and after, finding out the best condition of fermentation.
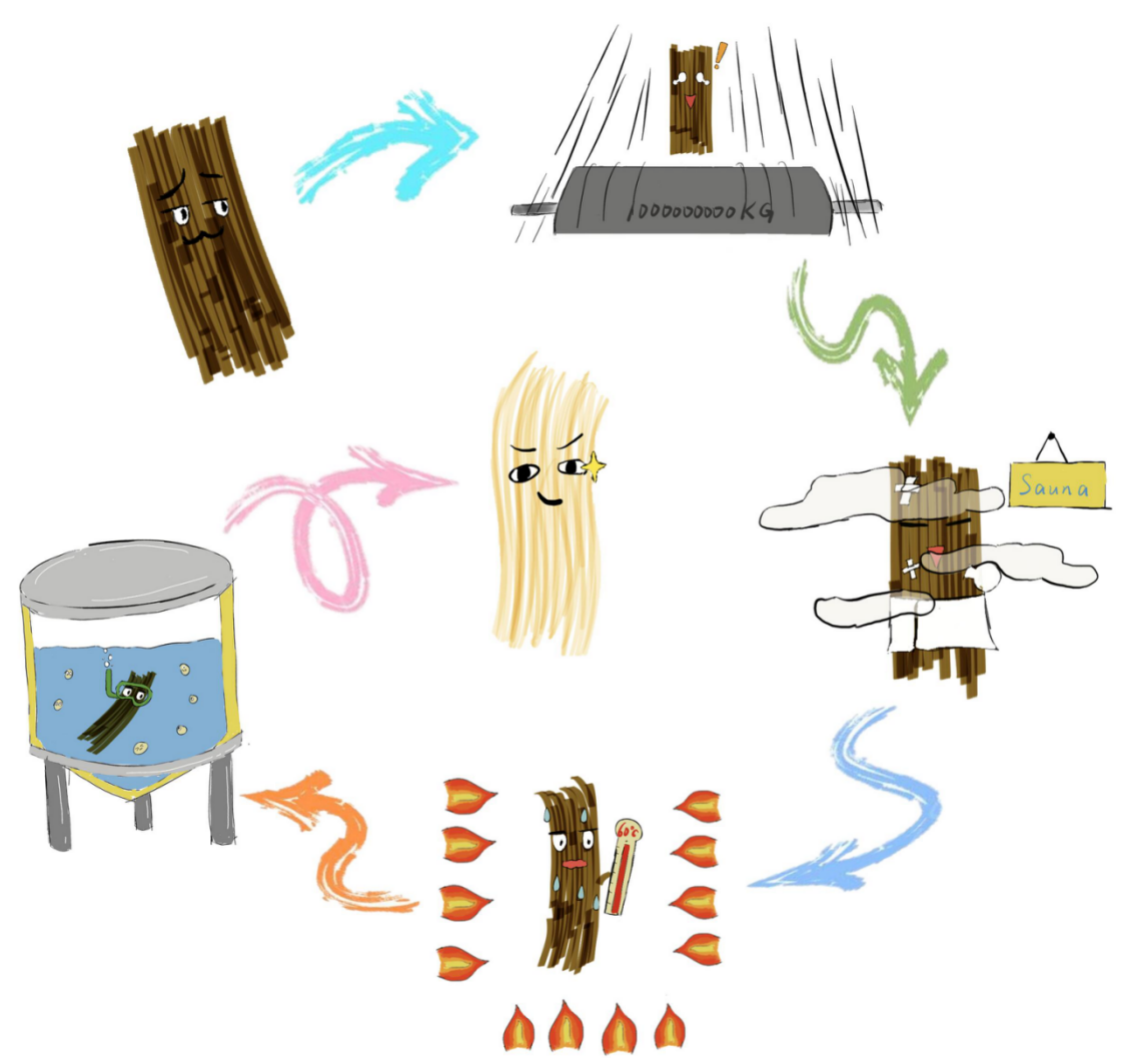
Reference: [1] Pei Fan. Research and application of in-situ biodegumming technology for ramie fiber[D].Hubei Huazhong University of Science and Technology, 2015. DOI:10.7666/d.D731116.

Reference: [1] Pei Fan. Research and application of in-situ biodegumming technology for ramie fiber[D].Hubei Huazhong University of Science and Technology, 2015. DOI:10.7666/d.D731116.





