In this section, we detail what our
objectives for our project are, and show what
considerations and experimental setups we conceived in order to
demonstrate those objectives.
Our project revolves around the
lengthening of bacterial functional lifespan, and by extension
refined control over bacterial metabolism. As such, we have set our objectives as follows:
- 1) Extend lifespan of engineered cells while imposing minimal changes to their existing designed functionalities
- 2) Retain better protein production capabilities after a prolonged period of time compared to cells without our system
- 3) Control over protein production with a varied range of inducers
- 4) Retention of system in the cell without antibiotic selection
With these objectives in mind, we detail our experimental design in the following sections.
Selection and Characterization of Toxin-Antitoxin Systems
In our project, we aimed to show the
extension of the functional lifespan of engineered bacteria, as well as the ability to control the activity of the cells. In order to do this, our first and primary method of cellular activity indication was that of OD
600. Bacterial growth rates are usually measured via the level and the rate of OD
600 increase over time. As such, we theorized that when cells are put to sleep, the OD
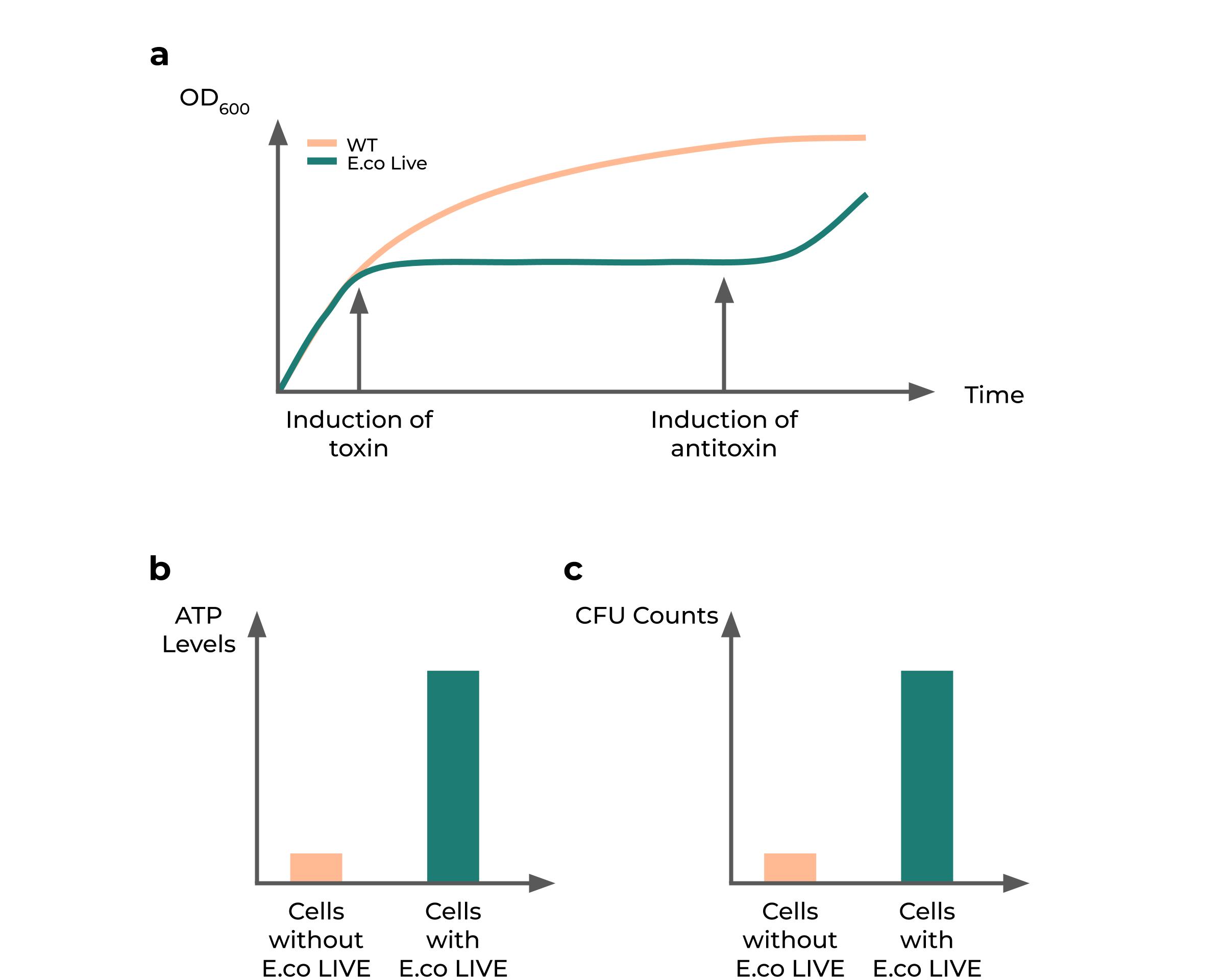
600 measurement should therefore stagnate and plateau for the period of time the cells are put to sleep. We therefore decided to use various toxin-antitoxin systems such as the HicA-HicB and the RES-Xre systems to achieve this goal.
We designed our toxin-antitoxin circuits as shown in the figure below. These proteins were
controlled by various chemical inducers, which in theory could be swapped out for
different kinds of inducers depending on the desired application.

Fig. 1: Circuit design of toxin-antitoxin systems (a) HicA-HicB and (b) RES-Xre.
We also hypothesized that since these toxins have a
bacteriostatic effect, the
growth could therefore be rescued with the external expression of the corresponding antitoxin. We therefore conducted multiple experiments with differing inducer concentrations, using our varying systems, to observe the change in the OD
600. This allowed us to gain a deep understanding of the quantity of toxins and antitoxins required to induce stasis and awaken the cells respectively. At the same time, we decided to
build a model that could explain the effect of the toxin and antitoxin on growth, which gave us some initial values of
relevant inducer concentrations to be used. We subsequently fed our experimental data back into the model to
build a more robust and representative one.
However, we also understood that a plateau in OD
600 could simply mean that cells are dying or dead - which is not an unusual observation due to the fact that some toxin-antitoxin systems have shown to be bacteriocidal, such as the Hok-Sok system and the Txe-Axe system (Fedorec et al., 2019). Thus, we decided to conduct experiments to
show that the cell was still alive. We used
CFU counts to show that our cells are still alive and are able to form colonies post-recovery with antitoxins. We further posited that if the toxin systems were bacteriostatic and supported the cell against metabolic stresses, there would be a higher proportion of live cells in experimental groups which have the toxin induced over time compared to that of normal fast-growing cells. These were similarly measured via CFU counts.
Since we hypothesize that a
lowered cellular resource consumption rate is what causes the plateau in growth, we posited that
dormant cells would contain a higher level of ATP compared to active cells. This is so as active cells consume significant amounts of ATP for protein production, which does not occur in growth-arrested cells. Thus, we decided to measure ATP in cells as a proxy for cellular resource consumption in the cell, and designed experiments comparing uninduced cells with their toxin-induced counterparts, using the BacTiter-Glo
™ kit from Promega to measure and determine the
extent of ATP accumulation in both samples over time.

Fig. 2: Hypothesized results of the effect of toxin-antitoxin systems on growth. (a) Predicted growth curve upon toxin induction and subsequent induction of antitoxin compared to normal growing cells. (b) Predicted accumulation of ATP over time in toxin-induced cells. (c) Predicted proportion of population being alive in cells having growth arrest compared to cells without arrest after 10 days.
Demonstration of cellular activity resumption after dormancy
In order to understand whether our toxin-antitoxin systems can truly prolong the viable lifespan of bacteria, we decided to understand if the
growth resumption upon antitoxin induction resulted in the cells performing its expected function, which, in the case of engineered cells, would be that of production of a desired protein. To test the hypothesis that the growth of the cells are correlated with protein production, we decided to utilize the commonly used
bioluminescence reporter gene, LuxCDABE, for our experiments.
LuxCDABE, being an operon with a significant number of genes and high metabolic burden, can only be induced for a short amount of time, and inducing cells which have already been starved for a period of time drastically reduces the bioluminescence strength and duration (Dunlap, 2009). Thus, by keeping the cells dormant, the
baseline nutrient consumption would drop, thus
prolonging the period in which the cells can be induced to produce a higher intensity and duration of bioluminescence compared to cells which have been kept awake. This would therefore demonstrate that the dormancy induced by the toxin can be recovered from, and does not subsequently affect protein production.
We similarly decided to test if our hypothesis would work across
different receptor and product outcomes. Therefore, we cloned in the quorum sensing circuit for
P. aeruginosa, and ran it through a similar experimental setup as our bioluminescence test. We observed the response of our engineered cells over a long period of time towards AHL induction by measuring RFP production. This gave us the chance to truly test the
versatility of our genetic circuit design.


Fig. 3: Coupling of growth switch with protein production. (a) Hypothesized results of the effect of toxin-antitoxin systems on protein production. (b) Genetic circuit of growth switch with LuxCDABE operon. (c) Genetic circuit of growth switch with AHL biosensor circuit.
Toggling between growth and dormancy
We also wanted to show that this state of dormancy and activity can be
reversible. It is shown in various studies that the effect of the toxin and antitoxin on the growth of the cell is largely determined by the ratio of toxin to antitoxin. If the toxin is higher, growth is inhibited and vice versa. As such, we hypothesized that by controlling the various levels of both proteins and specific timepoints, toggling between growth and dormancy could be achieved.
In order to do so, we needed a way to
tune the protein expression of the genes over time. We first utilized the ubiquitous chemical operons, the lactose and arabinose operons, to control the expression of our toxin and antitoxin systems respectively. This allowed us to characterize the effect of the toxin on growth, as well as understand how much antitoxin was required to neutralize the effect of its cognate toxin.
However, such
chemical systems would not be sufficient to test the effect of our toxin-antitoxin systems. This is because as chemical inducers are not reversible, and to test these multiple states would require multiple steps to wash away the inducer. This introduces further variability to our experiments, which already require significant care and precision due to how variable growth measurements of bacteria can be. Thus, we designed a
blue-light switch system, based on the EL222 repressible system, to control the expression of the antitoxins in the toxin-antitoxin pair (Jayaraman et al., 2016). The blue-light system in our design controls the expression of the antitoxin while being coupled to a chemically inducible toxin. It is designed such that
expression of the antitoxin would be repressed upon exposure of cells to blue light. This, coupled with the chemical induction of the toxin, would then result in the toxin:antitoxin ratio being higher than 1 and thereby resulting in repression of growth. The growth could then be recovered by placing the system in the dark, resulting in derepression of the antitoxin and thus recovery. We theorize that the light system would demonstrate the
versatility of our system and also allow us
better temporal control over gene expression.

Fig. 4: Alternative inducer control circuits in the form of a (a) blue-light controlled circuit. (b) Hypothesized growth curve upon exposure to light for a specific period of time. Exposure to light results in arrest, while placing cells in the dark results in growth recovery.
In our experiments, we chose to work with mainly two toxin-antitoxin systems - namely the
HicA-HicB and RES-Xre systems. Of note is that
E. coli possesses an endogenous HicA-HicB operon, which poses implications for our experimental design. This is so as the endogenous HicA-HicB operon has been shown to possess its own unique regulatory pathways, which might interact with our exogenous HicA-HicB system in unpredictable ways. For example, native
E. coli HicA-HicB operon can be regulated by its own product - the HicA toxin stimulates the selective production of HicB, which would ultimately result in growth recovery (Turnbull & Gerdes, 2017). Our exogenously controlled system and models would be unable to take these understudied endogenous regulatory pathways into account. As such, to properly characterize the HicA-HicB system, we decided to use
HicA-HicB knockout strains generously provided by the Brodersen group. The RES-Xre system does not seem to have this problem due to it being orthogonal to
E. coli.
Showing tunability of growth using SgrS
Since our toxin-antitoxin systems were meant to show a distinct switching behavior between being ‘on’ or ‘off’, we decided to further expand our repertoire of growth control to include a more
continuous manner of growth control, much like a
volume knob compared to an ‘ON/OFF’ switch. As such, we designed the SgrS system that has been shown previously to have a growth inhibition effect. We controlled the level of SgrS using the tetracycline inducible system, due to the other chemical systems having a direct impact on the glucose metabolic pathway, which would result in unwanted variability in our experimental setup. We hypothesized that since
SgrS simply lowers the glucose transport into the cell, it would have a more
continuous and slope-like behavior than the toxin-antitoxin systems. As a result, the protein production curve would be similarly lower, resulting in a more sustained, albeit lower overall level of protein production. We therefore tested differing concentrations of inducer to determine exactly how continuous and/or linear the lowering of growth would be in relation to amount of SgrS being induced. We also designed experiments to
test the effect of SgrS on protein production to determine if our hypothesis of having a gentler protein production curve is true. To test the SgrS system, we used M9 media instead of Luria-Bertani Broth due to the effect of SgrS being closely linked to glucose concentration. Thus, M9 media, with a known initial concentration of glucose, was more suitable for SgrS characterization.

Fig. 5: Illustration of (a) growth curve changes upon increasing SgrS concentration, (b) protein production patterns upon increasing SgrS concentration, and (c) SgrS circuit.
To verify that glucose is indeed being consumed at a lower rate, we used the Glucose-Glo
™ kit from Promega to determine the glucose levels in the media. This allowed us to
quantify the different rates of glucose being consumed by cells with or without SgrS being induced. We hypothesized that since SgrS is inhibiting glucose intake, the
rate of glucose consumed will be slower for cells producing SgrS compared to those without SgrS.
Plasmid Retention & Biocontainment
To implement our plasmid retention cum biocontainment module, we were inspired by previous groups such as the iGEM 2014 Wageningen and iGEM 2016 Toulouse groups. We designed the system with two bacteriocidal toxin-antitoxin systems - the Txe-Axe system as well as the Hok-Sok system. The necessity of having to retain both plasmids serve both the our needs to retain the system in the cell, as well as reducing the change of plasmid uptake by external hosts - achieving both retetion and biocontainment. We noted the difficulties other teams faced in obtaining live and successfully transformed colonies, possibly due to the problems with maintaining an appropriate balance of toxins and antitoxins for survivability, and put significant thought into the design of our system. We kept the concept of keeping the
toxin and its cognate antitoxin on separate plasmids. However, to ensure that the antitoxin is significantly higher in concentration compared to the toxin and therefore increasing the chances of cellular survival, we placed a
strong promoter in front of the antitoxin and a corresponding, weaker promoter in front of the toxin. We theorized that this should allow the cells to produce sufficient antitoxins, even in a weakened state, compared to the cognate toxin and allow the cell to survive. Doing this also negated the possible effects of any endogenous regulatory elements as the toxin-antitoxin production would likely be solely controlled by the promoter strength. We also utilized two plasmids with very similar copy number strengths to ensure that this would not be a significant confounding factor in our experiments.
In order to test whether this system indeed prolongs the retention of our desired plasmid, we conceptualized experiments utilizing reporter proteins to test how long the plasmid is retained compared to systems with just one toxin-antitoxin system as well as standard plasmid vector backbones. One experiment would be to transform cells with both plasmids simultaneously, yet plating them on plates
only selecting for one plasmid. Cells would have to retain both plasmids even on plates containing just one selection factor, as the enforced retention of one plasmid with its respective antibiotic would necessitate retention of the other plasmid due to the presence of the toxin in that plasmid. As a result, both plates should retain a large proportion of cells containing both plasmids, as compared to a dual transformation of plasmids without this system on a single selection media.
To test the biocontainment aspect of the system would be more difficult, due to time constraints and the nature of our circuit design. Nevertheless, we envision that teams in the future can increase safety of the module by removing the antibiotic selection markers post-transformation, and transform single plasmids to test the efficacy of the bacteriocidal effect. Microbial consortia experiments can also be set up to determine the genetic transfer efficiency and thus the biocontainment effect of the circuit.

Fig. 6: Illustrations of (a) genetic circuit design for dual plasmid retention system and (b) expected result upon plating of co-transformed bacteria on a plate selecting for one of the 2 retention plasmids, compared to co-transformation without the retention system.
References
Dunlap, P. V. (2009). Bioluminescence, Microbial. In Encyclopedia of Microbiology. https://doi.org/10.1016/b978-012373944-5.00066-3
Fedorec, A. J. H., Ozdemir, T., Doshi, A., Ho, Y. K., Rosa, L., Rutter, J., … Barnes, C. P. (2019). Two New Plasmid Post-segregational Killing Mechanisms for the Implementation of Synthetic Gene Networks in Escherichia coli. Food Science and Human Wellness. https://doi.org/10.1016/j.isci.2019.03.019
Turnbull, K. J., & Gerdes, K. (2017). HicA toxin of Escherichia coli derepresses hicAB transcription to selectively produce HicB antitoxin. Molecular Microbiology. https://doi.org/10.1111/mmi.13662